Engineers are building a treatment system that they can tune to selectively pull toxins from drinking water and wastewater from factories, sewage systems, and oil and gas wells. They say the technology will cut costs and save energy compared to conventional systems.
“Traditional methods to remove everything, such as reverse osmosis, are expensive and energy intensive,” says Qilin Li, a professor of civil and environmental engineering and of materials science and nanoengineering at Rice University. “If we figure out a way to just fish out these minor components, we can save a lot of energy.”
“There are a lot of ions in water. Not everything is toxic.”
The heart of the system is a set of new composite electrodes that allow capacitive deionization. The charged, porous electrodes selectively pull target ions from fluids passing through the maze-like system. When the pores fill with toxins, the electrodes can be cleaned, restored to their original capacity, and reused.
“This is part of a broad scope of research to figure out ways to selectively remove ionic contaminants,” says Li, the lead scientist and coauthor of the study in Environmental Science & Technology. “There are a lot of ions in water. Not everything is toxic. For example, sodium chloride (salt) is perfectly benign. We don’t have to remove it unless the concentration gets too high.
“For many applications, we can leave non-hazardous ions behind, but there are certain ions that we need to remove,” she says. “For example, in some drinking water wells, there’s arsenic. In our drinking water pipes, there could be lead or copper. And in industrial applications, there are calcium and sulfate ions that form scale, a buildup of mineral deposits that foul and clog pipes.”
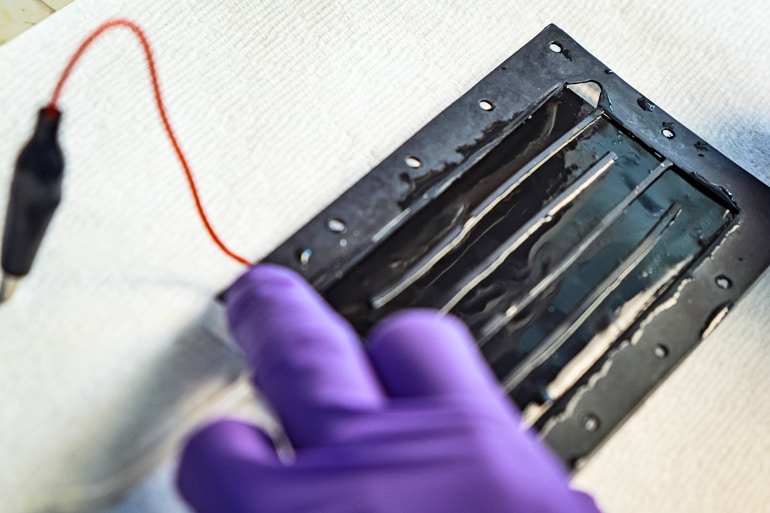
The proof-of-principal system removed sulfate ions, a scale-forming mineral that can give water a bitter taste and act as a laxative. The system’s electrodes were coated with activated carbon, which was in turn coated by a thin film of tiny resin particles held together by quaternized polyvinyl alcohol. When sulfate-contaminated water flowed through a channel between the charged electrodes, sulfate ions were attracted by the electrodes, passed through the resin coating and stuck to the carbon.
Lab tests showed the positively charged coating on the cathode preferentially captured sulfate ions over salt at a ratio of more than 20 to 1. The electrodes retained their properties over 50 cycles.
“But in fact, in the lab, we’ve run the system for several hundred cycles and I don’t see any breaking or peeling of the material,” says lead author Kuichang Zuo, a postdoctoral researcher in Li’s lab. “It’s very robust.”
Nature, not just industry, puts toxic chromium into water
The team intends the system to work with current commercial water-treatment systems, Li says. “The true merit of this work is not that we were able to selectively remove sulfate, because there are many other contaminants that are perhaps more important,” she says. “The merit is that we developed a technology platform that we can use to target other contaminants as well by varying the composition of the electrode coating.”
The team is developing coatings for other contaminants and working with labs at the University of Texas at El Paso and Arizona State University on large-scale test systems. Zuo says it should also be possible to scale systems down for in-home water purification.
Device makes clean water with paper and sunlight
Additional coauthors are from Rice and from Shanghai Jiao Tong University in China. The National Science Foundation-backed Rice-based Center for Nanotechnology-Enabled Water Treatment, the Welch Foundation, and the Shanghai Municipal International Cooperation Foundation funded the work.
Source: Rice University



