Researchers have created an efficient, low-cost device that splits water to produce hydrogen fuel.
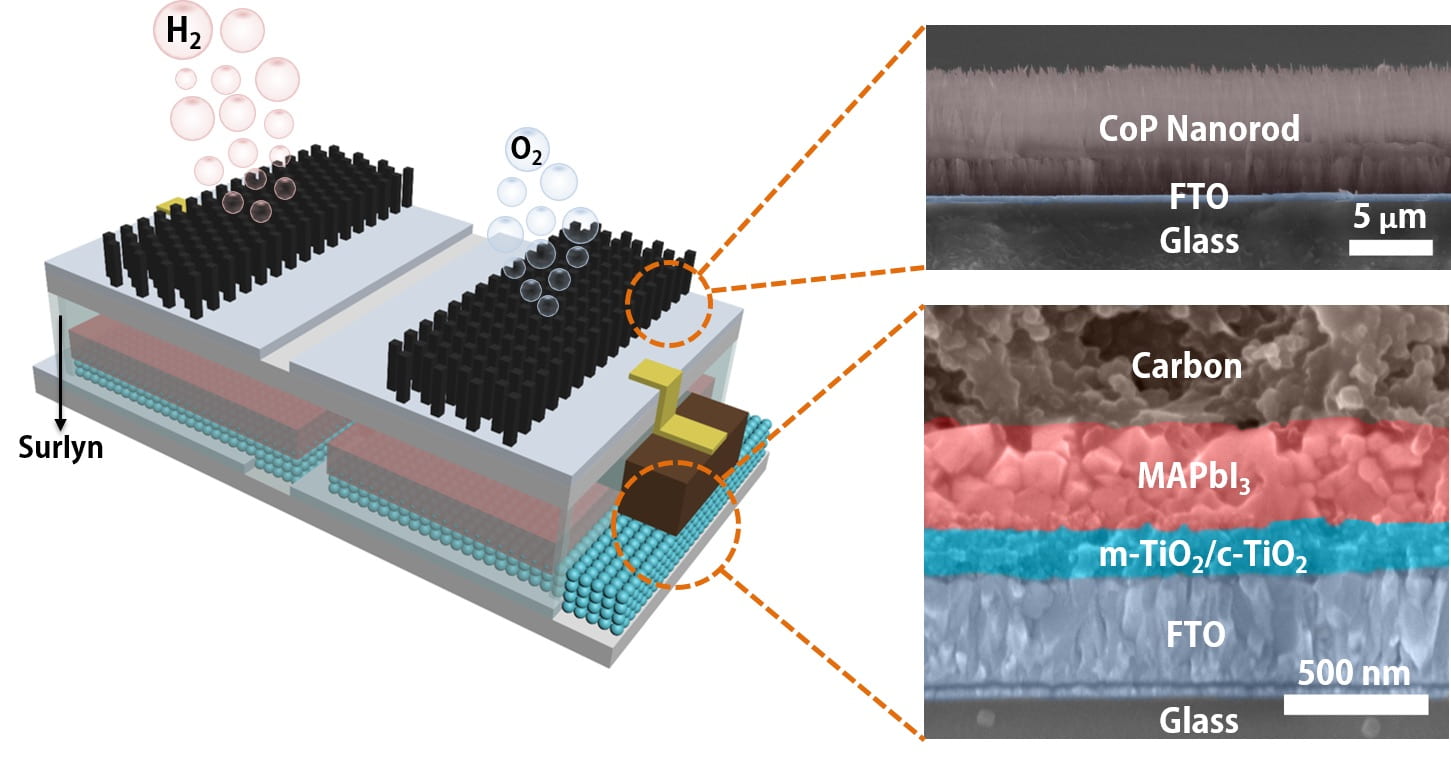
The platform integrates catalytic electrodes and perovskite solar cells so that, when sunlight triggers it, the device produces electricity. The current flows to the catalysts that turn water into hydrogen and oxygen, with a sunlight-to-hydrogen efficiency as high as 6.7%.
“With a clever system design, you can potentially make a self-sustaining loop.”
This sort of catalysis isn’t new, but the lab packaged a perovskite layer and the electrodes into a single module that, when dropped into water and placed in sunlight, produces hydrogen with no further input.
The platform is a self-sustaining producer of fuel that, the researchers say, should be simple to produce in bulk.
“The concept is broadly similar to an artificial leaf,” says Jun Lou, a professor of materials science and nanoengineering and of chemistry, whose lab in the Brown School of Engineering at Rice University developed the platform.
“What we have is an integrated module that turns sunlight into electricity that drives an electrochemical reaction. It utilizes water and sunlight to get chemical fuels.”
Perovskites are crystals with cubelike lattices that harvest light. The most efficient perovskite solar cells produced so far achieve an efficiency above 25%, but the materials are expensive and light, humidity, and heat tend to stress them.
“Jia has replaced the more expensive components, like platinum, in perovskite solar cells with alternatives like carbon,” Lou says. “That lowers the entry barrier for commercial adoption. Integrated devices like this are promising because they create a system that is sustainable. This does not require any external power to keep the module running.”
The key component may not be the perovskite but the polymer that encapsulates it, protecting the module and allowing to be immersed for long periods, says lead author Jia Liang, a postdoctoral fellow.
“Others have developed catalytic systems that connect the solar cell outside the water to immersed electrodes with a wire,” he says. “We simplify the system by encapsulating the perovskite layer with a Surlyn (polymer) film.”
The patterned film allows sunlight to reach the solar cell while protecting it and serves as an insulator between the cells and the electrodes, Liang says.
“With a clever system design, you can potentially make a self-sustaining loop,” Lou says. “Even when there’s no sunlight, you can use stored energy in the form of chemical fuel. You can put the hydrogen and oxygen products in separate tanks and incorporate another module like a fuel cell to turn those fuels back into electricity.”
The researchers say they will continue to improve the encapsulation technique as well as the solar cells themselves to raise the efficiency of the modules.
The research appears in ACS Nano.
Additional coauthors are from Rice University; Northwestern Polytechnical University in Xi’an, China; Washington University in St. Louis; and Tsinghua University. Funding for the research came from Rice’s Smalley-Curl Institute; the Welch Foundation; the National Science Foundation-backed Nanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment; and Fundamental Research Funds for the Central Universities, China.
Source: Rice University



