Personalized 3D heart simulations can accurately identify tissue doctors should electrically destroy to stop potentially fatal irregular, rapid heartbeats.
A retrospective analysis of 21 patients with ventricular tachycardia and a prospective study of five establishes that 3D simulation-guided procedures are worthy of expanded clinical trials, researchers say.
“Cardiac ablation, or the destruction of tissue to stop errant electrical impulses, has been somewhat successful but hampered by a lot of guesswork and variability in the way that physicians figure out which locations to zap,” says Natalia Trayanova, professor of biomedical engineering at Johns Hopkins University.
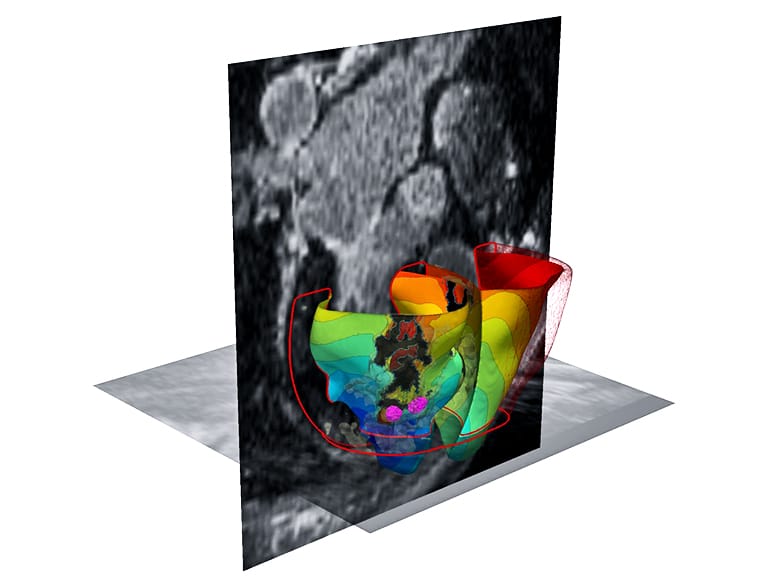
The new study suggests “we can remove a lot of the guesswork, standardize treatment, and decrease the variability in outcomes, so that patients remain free of arrhythmia in the long term,” she says.
Relax and refill
When a normal heart contracts to pump blood through the body, a wave of electrical signals flows through the muscle, stimulating each cardiac cell to contract—one after the other—in a steady rhythm. After the heart contracts, it relaxes and refills with blood.
In ventricular tachycardia, electrical signals in the heart’s lower chambers misfire, crippling the relaxation and refilling process and producing rapid, irregular pulses. These arrhythmias are linked to an estimated 300,000 sudden cardiac deaths in the United States each year.
Drugs can manage so-called infarct-related ventricular tachycardia, but drug side effects and limitations have increased focus on the potential of cardiac ablation. It essentially “rewires” electrical signaling that gives rise to arrhythmia.
Trayanova says current estimates are that cardiac ablation is successful between 50 and 88 percent of the time, but outcomes are difficult to predict.
How heart cells behave
In a traditional ablation, doctors thread a catheter through blood vessels to reach the heart and use radiofrequency waves to destroy regions of heart tissue believed to sustain and propagate erratic electrical waves. Mapping of the heart’s electrical functioning with a catheter is used to locate likely problem areas, but precise pinpointing has been a challenge.
To locate arrhythmias more precisely, Trayanova and her research team developed 3D personalized computational models of patient hearts, based on contrast-enhanced clinical MRI images. Each heart tissue cell in the model generates electrical signals with the aid of mathematical equations representing how heart cells behave when they are healthy or when they are semi-viable when near the scar.
By poking a patient’s virtual heart with small simulated electrical signals, the computer program determines whether the heart develops an arrhythmia and locates the tissue that perpetuates it. Trayanova then simulates an ablation to that area and runs the computer program repeatedly to find multiple locations that doctors should ablate on the actual patient.
The reseachers used MRI images to create personalized heart models of 21 people who previously had successful cardiac ablation for infarct-related ventricular tachycardia.
3D modeling of these patients correctly predicted the locations where physicians in real life had ablated heart tissue. In fact, in five patients, the amount of ablated tissue the 3D model identified was smaller than the area the actual procedures destroyed.
Fewer complications
Next, the researchers tested 3D simulation to guide cardiac ablation for three patients at the University of Utah and two at the University of Pennsylvania. Two patients who received simulation-guided ablation have remained free of tachycardia for 23 and 21 months, respectively.
One patient remained free of tachycardia after two months of follow up. In two patients, the virtual heart approach predicted that tachycardias would not be inducible. That was confirmed during the clinical procedure, so cardiac ablation was not performed.
The study represents the first attempt to incorporate personalized simulation into arrhythmia treatment. The researchers believe the procedure will cut down the lengthy and invasive cardiac mapping process and reduce complications. The technology could also reduce the need for repeat procedures.
“It’s an exciting blend of engineering and medicine,” Trayanova says.
Further clinical trials are necessary to validate the procedure’s promise, Trayanova says. The Food and Drug Administration recently approved clinical study at Johns Hopkins.
The National Institutes of Health funded the study, which appears in Nature Biomedical Engineering.
Source: Johns Hopkins University


