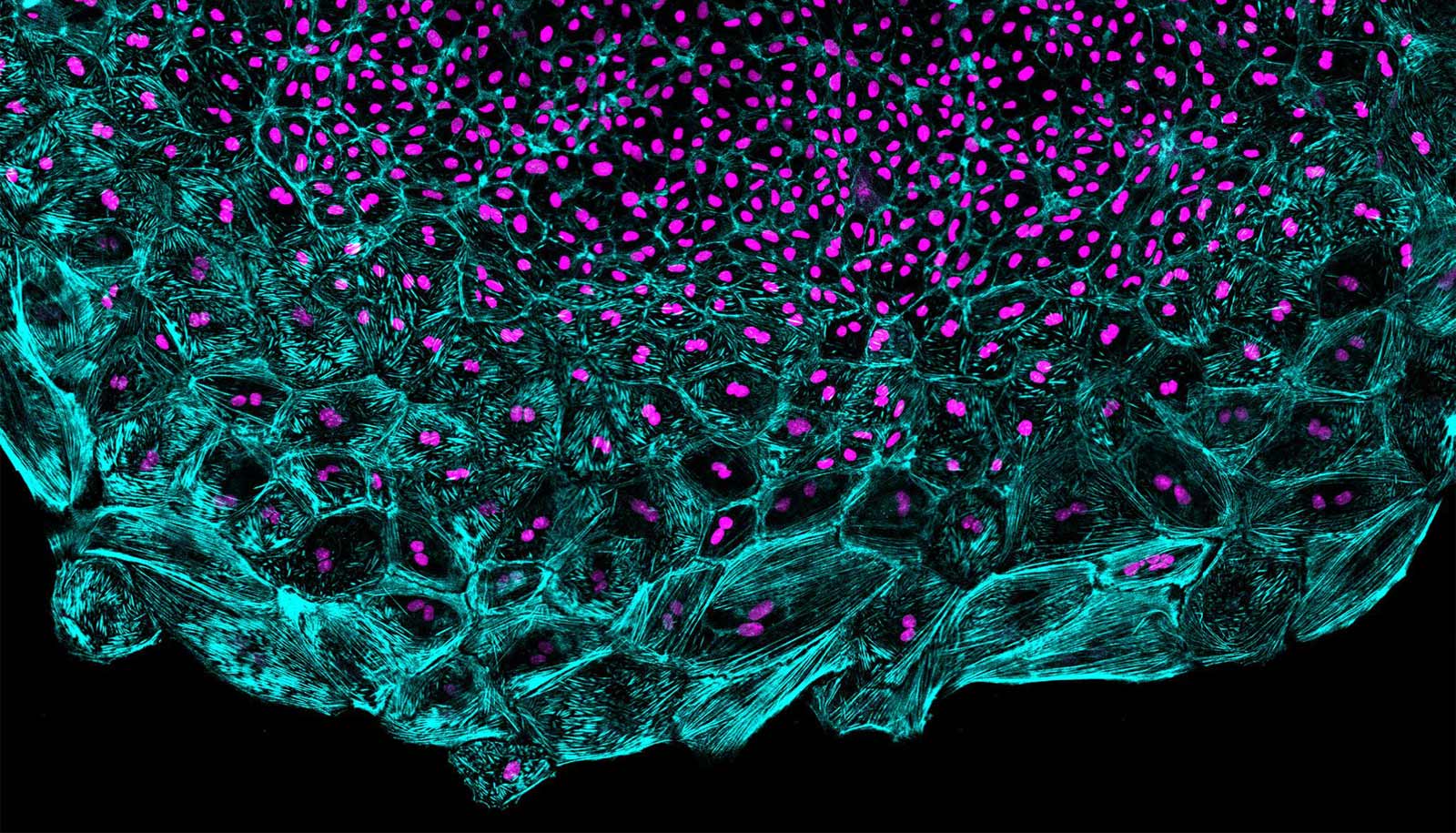
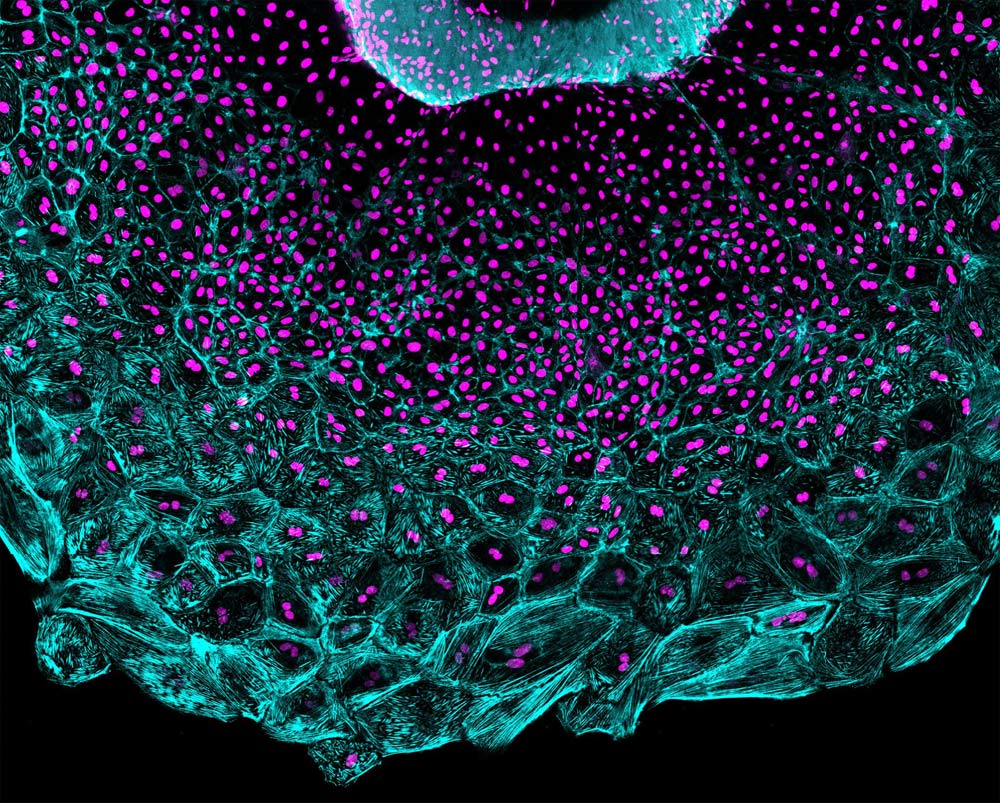
By taking videos of a tiny beating zebrafish heart as it reconstructs its covering in a petri dish, scientists have captured unexpected dynamics of cells involved in tissue regeneration.
They found that the depleted heart tissue regenerates itself in a wave, led by a front of fast-moving, supersized cells and trailed by smaller cells that multiply to produce others.
The nature of this wavefront—and the success of the tissue regeneration that follows—is determined by mechanical tension that acts upon the cells. The results, published in the journal Developmental Cell, indicate a new paradigm for how forces acting in tissues can direct the decisions that cells make to regenerate lost tissues.
“Our findings open avenues for the study of cell cycle dynamics in regenerating tissue,” says Jingli Cao, lead study author and a postdoctoral fellow in Ken Poss’s lab at Duke University School of Medicine. “By manipulating the mechanical tension of cells, we also might be able to develop new bioengineering or translational approaches.”
While the human heart can’t fully heal itself after a heart attack, the zebrafish heart can easily replace cells lost to damage or disease. Scientists have spent many years probing the regenerative powers of this small striped fish in the hopes of uncovering clues that could improve therapy for human heart disease.
In 2015, Cao showed that he could remove the hearts from zebrafish and grow them in dishes in the laboratory, where the tiny two-chambered organs continued to beat and behave as if they were still tucked inside the animal. In this study, Cao and his colleagues exploited this system to monitor the regeneration of the epicardium, a thin layer of cells that cover the heart’s surface.
The researchers destroyed most of the heart’s epicardial layer, and then put the “explanted” organs under the microscope to capture the regeneration in action. They expected to see a population of cells that had rapidly replicated their DNA content and divided into new cells replenish the surface of the organ. While these cells definitely played a part, they were not leading the charge. Instead, regeneration was led by cells that replicated their DNA without dividing, effectively creating supersized cells with two times the cell machinery or more.
“Imagine you have a wound on your skin and you want to cover it as soon as possible, but you don’t have enough cells,” says Cao. “By making cells become larger, you could efficiently cover the wound. We think this tactic could increase the regenerative capacity of this population by covering the surface in an efficient manner.”
The researchers measured a number of properties of the cells in the regenerative wavefront. They found that the bigger leader cells migrated across the surface of the heart at higher speeds than the smaller follower cells. When they measured the levels of tension experienced by the cells, they found that leader cells recoiled faster than follower cells when tiny incisions were applied, much like the surface of an inflated balloon retracts after bursting. Poss says that mechanical tension seems to keep the cells from dividing after DNA replication.
“This study is trying to understand the basic decisions cells make when they regenerate,” says Poss, professor of cell biology at Duke University School of Medicine and director of the Regeneration Next Initiative at Duke. “If there are methods we could use to guide their decisions, to determine whether they generate larger cells or more cells through division, it could be one way to influence the ability of a tissue to repair.”
‘Origami organs’ could regenerate tissue
The researchers plan to use their zebrafish heart explant culture system to screen for small molecules that could potentially increase the regenerative capacity of heart tissues. Such chemicals could one day form the basis for new drugs to repair the damage caused by a heart attack or other cardiovascular diseases.
This study was a collaboration among several research groups at Duke that Poss, Stefano Di Talia, Daniel Kiehart, and Nenad Bursac lead, as well as Eric Small’s group at University of Rochester. The American Heart Association, the National Science Foundation, the National Institutes of Health, and the Leducq Foundation supported the work.
Source: Duke University