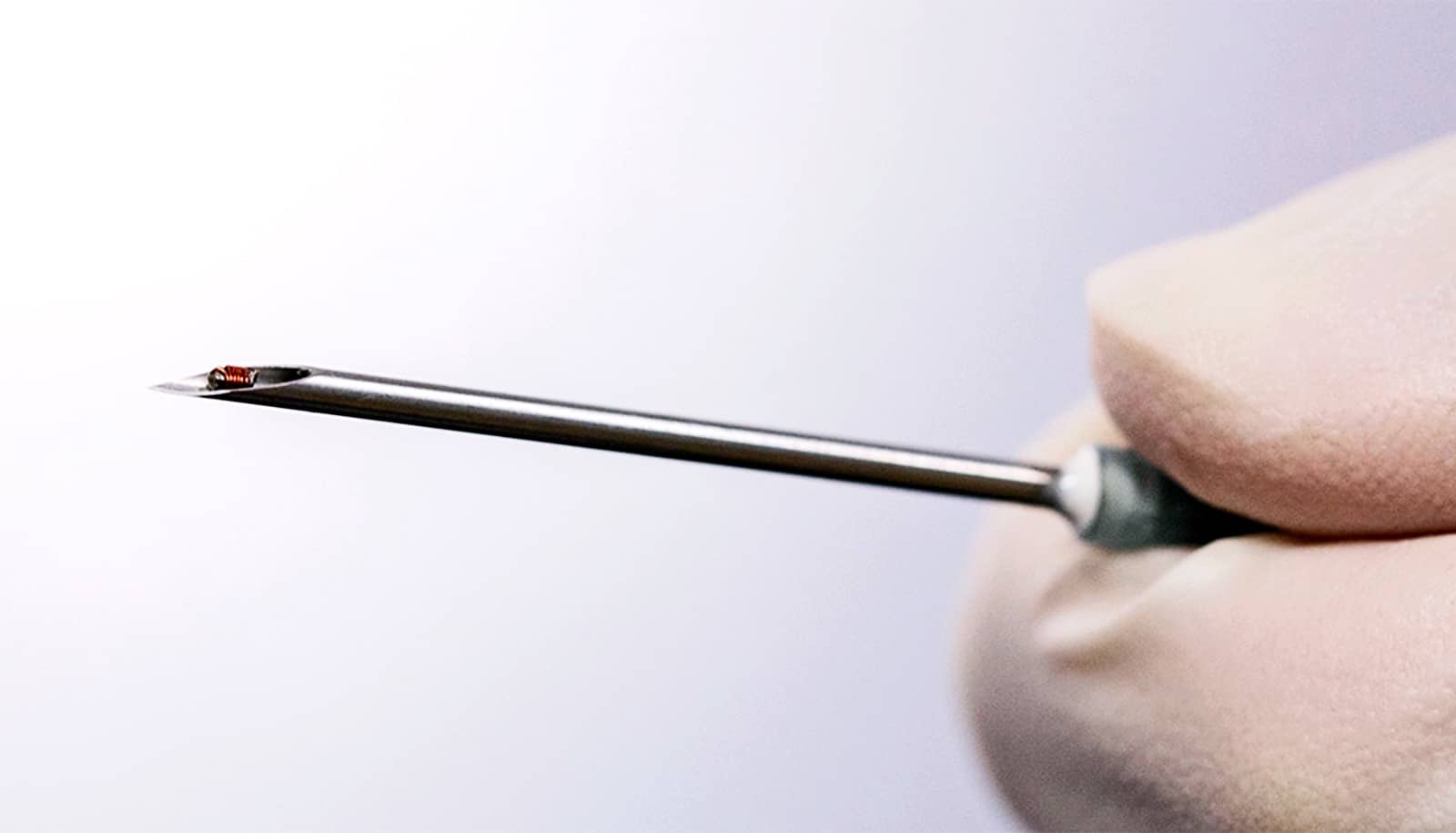
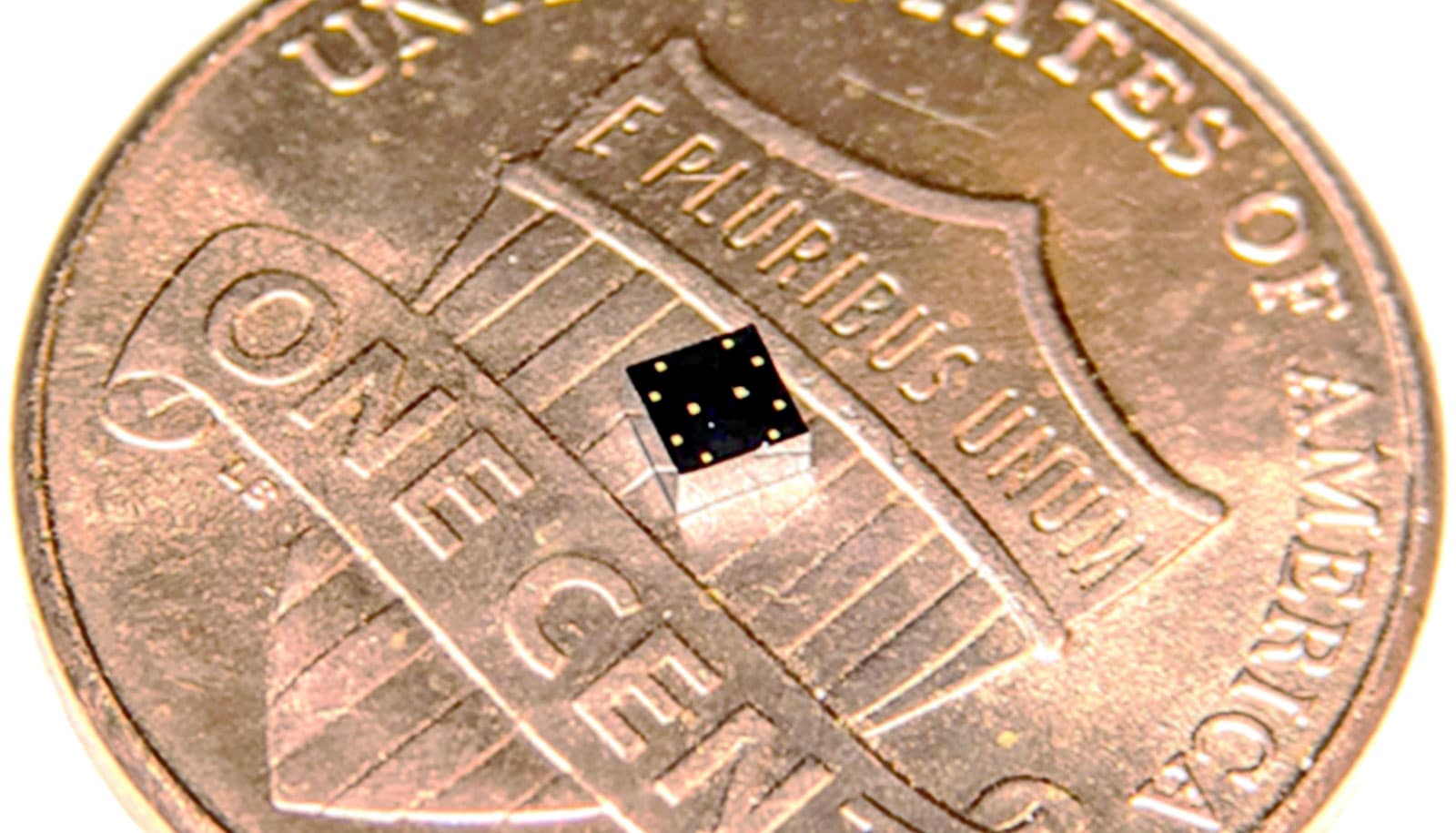
An implant not much bigger than a grain of rice could treat chronic pain and diseases and neurological diseases by stimulating nerves.
Researchers have published the first proof-of-concept results from a years-long program to develop tiny, wireless devices that can treat neurological diseases or block pain.
The nerve stimulators require no batteries and instead draw both their power and programming from a low-powered magnetic transmitter outside the body.
“Because the devices are so small, we can use blood vessels as a highway system to reach targets that are difficult to get to with traditional surgery.”
The MagnetoElectric Bio ImplanT—aka ME-BIT—is placed surgically and an electrode is fed into a blood vessel toward the nerve targeted for stimulation. Once there, the device can be powered and securely controlled with a near-field transmitter worn close to the body.
Researchers successfully tested the technology on animal models and found it could charge and communicate with implants several centimeters below the skin.
The implant, detailed in Nature Biomedical Engineering, could replace more invasive units that now treat Parkinson’s disease, epilepsy, chronic pain, hearing loss, and paralysis.
“Because the devices are so small, we can use blood vessels as a highway system to reach targets that are difficult to get to with traditional surgery,” says Jacob Robinson, an associate professor of electrical and computer engineering and of bioengineering at Rice University.
“We’re delivering them using the same catheters you would use for an endovascular procedure, but we would leave the device outside the vessel and place a guidewire into the bloodstream as the stimulating electrode, which could be held in place with a stent.”
No batteries required
The ability to power the implants with magnetoelectric materials eliminates the need for electrical leads through the skin and other tissues. Leads like those often used for pacemakers can cause inflammation, and sometimes need to be replaced. Battery-powered implants can also require additional surgery to replace batteries.
ME-BIT’s wearable charger requires no surgery. The researchers showed it could even be misaligned by several inches and still sufficiently power and communicate with the implant.
The programmable, 0.8-square-millimeter implant incorporates a strip of magnetoelectric film that converts magnetic energy to electrical power. An on-board capacitor can store some of that power, and a “system-on-a-chip” microprocessor translates modulations in the magnetic field into data. The components are held together by a 3D-printed capsule and further encased in epoxy.
The magnetic field generated by the transmitter—about 1 milliTesla—is easily tolerated by tissues, the researchers say. They estimate the current implant can generate a maximum of 4 milliwatts of power, sufficient for many neural stimulation applications.
“One of the nice things is that all the nerves in our bodies require oxygen and nutrients, so that means there’s a blood vessel within a few hundred microns of all the nerves,” Robinson says. “It’s just a matter of tracing the right blood vessels to reach the targets.
“With a combination of imaging and anatomy, we can be pretty confident about where we place the electrodes,” he says.
Real-time sensing
The research suggests endovascular bioelectronics like ME-BIT could lead to a wide range of low-risk, highly precise therapies. Having electrodes in the bloodstream could also enable real-time sensing of biochemical, pH, and blood-oxygen levels to provide diagnostics or support other medical devices.
The team ultimately hopes to employ multiple implants and communicate with them simultaneously, Robinson says.
“That way we could have a distributed network at multiple sites. Other things we’re looking to add are sensing, recording, and back-channel communications so we can use the implants to both record and stimulate activity as part of a closed system.”
Kaiyuan Yang, an assistant professor of electrical and computer engineering at Rice, and Sunil Sheth, an associate professor and director of the vascular neurology program at McGovern Medical School of the University of Texas Health Science Center, are co-lead authors of the study.
The National Institutes of Health and the National Science Foundation supported the research.
Source: Rice University