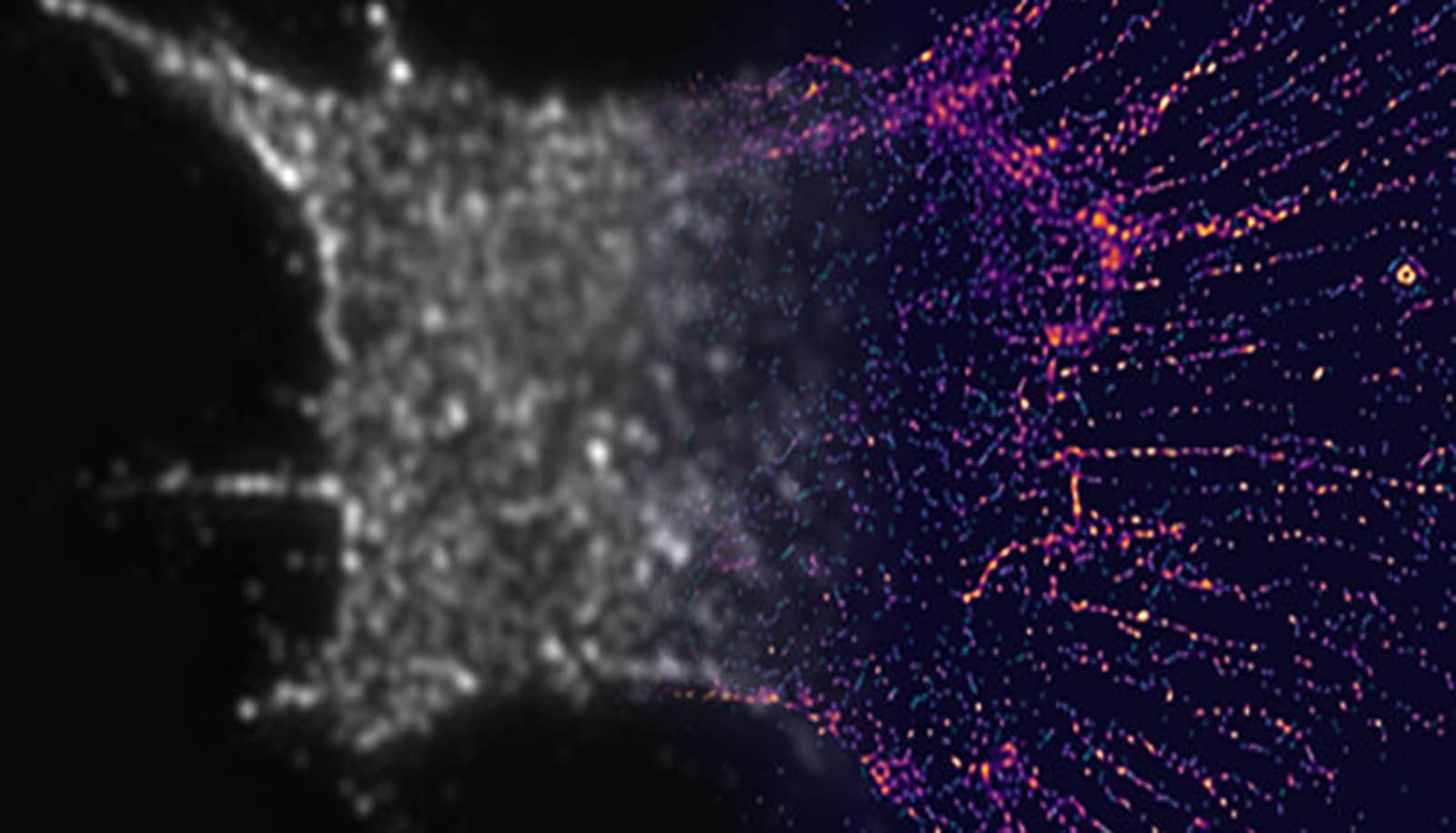
A new way to see details smaller than half the wavelength of light has revealed how nanoscale scaffolding inside cells bridges to the macroscale during cell division.
Unlike earlier superresolution techniques, this one doesn’t rely on molecules that wear out with prolonged use.
Superresolution can reveal structures down to 10 nanometers, or about the same breadth as 100 atoms. It opened a whole new world in biology, and the techniques that first made it possible received a Nobel Prize in 2014. However, its weakness is that it can only take snapshots over tens of seconds. This makes it impossible to observe the evolution of the machinery of a cell over long periods of time.
“We were wondering—when the system as a whole is dividing, how do nanometer-scale structures interact with their neighbors at the nanometer scale, and how does this interaction scale up to the whole cell?” says Somin Lee, assistant professor of electrical and computer engineering at the University of Michigan, who led the study published in Nature Communications.
To answer that question, Lee and colleagues needed a new kind of superresolution. Using their new method, they were able to continuously monitor a cell for 250 hours.
“The living cell is a busy place with proteins bustling here and there. Our superresolution is very attractive for viewing these dynamic activities,” says Guangjie Cui, a PhD student in electrical and computer engineering and co-first author of the study with Yunbo Liu, a PhD graduate in electrical and computer engineering.
Like the original method, the new technique uses probes near the nanoscale objects of interest to shed light on them. Superresolution 1.0 used fluorophores for this, fluorescent molecules that would send out an answering light after being illuminated. If the fluorophores were closer together than the size of whatever was being imaged, the image could be reconstructed from the bursts of light produced by the fluorophores.
The new technique uses gold nanorods, which don’t break down with repeated exposure to light, but making use of the light that interacts with them is more challenging. Nanorods respond to the phase of the light, or where it is in the up-and-down oscillation of the electric and magnetic fields that compose it. This interaction depends on how the nanorod is angled to the incoming light.
Like the fluorophores, the nanorods can attach to particular cell structures with targeting molecules on their surfaces. In this case, the nanorods sought out actin, a protein that adds structure to soft cells. Actin is shaped like branching filaments, each about 7 nanometers (millionths of a millimeter) in diameter, though they link together to span thousands of nanometers. Even though the nanorods are often more than twice the diameter of the actin, the data they provide as a group can illuminate its tiny details.
To locate the nanorods, the team built filters made of thin layers of polymers and liquid crystals. These filters enabled the detection of light with a particular phase, enabling the team to pick out nanorods with particular angles to the incoming light. By taking 10 to 30 images—each looking at a different subset of nanorods—and merging them into a single image, the team was able to deduce the nanometer-scale details of the filaments inside the cells. These details would be blurred out in conventional microscopes.
Using the technique, the team discovered three rules governing the way that actin self-organizes during cell division:
- Actin expands to reach its neighbors when actin filaments are far apart.
- Actin will draw nearer to its neighbors to increase connections, although this tendency is tempered by the drive to expand and reach more neighbors.
- As a result, the actin network tends to contract when it is more connected, and it will expand when it is less connected.
The behavior of the actin is connected to the behavior of the cell—but the cell contracts when the actin expands, and it expands when the actin contracts. The team wants to explore this further, discovering why the motions are opposite at different scales. They also want to investigate the consequences of dysregulating this molecular process: Is this at the root of some diseases?
More broadly, they hope to use superresolution to understand how self-organization is built into biological structures, without the need for central control.
“Our genetic code doesn’t actually include enough information to encode every detail of the organization process,” Lee says. “We want to explore the mechanisms of collective behaviors without central coordination that are like birds flying in formation—in which the system is driven by interactions between individual parts.”
The study had support from the Air Force Office of Scientific Research and the National Science Foundation.
Source: University of Michigan