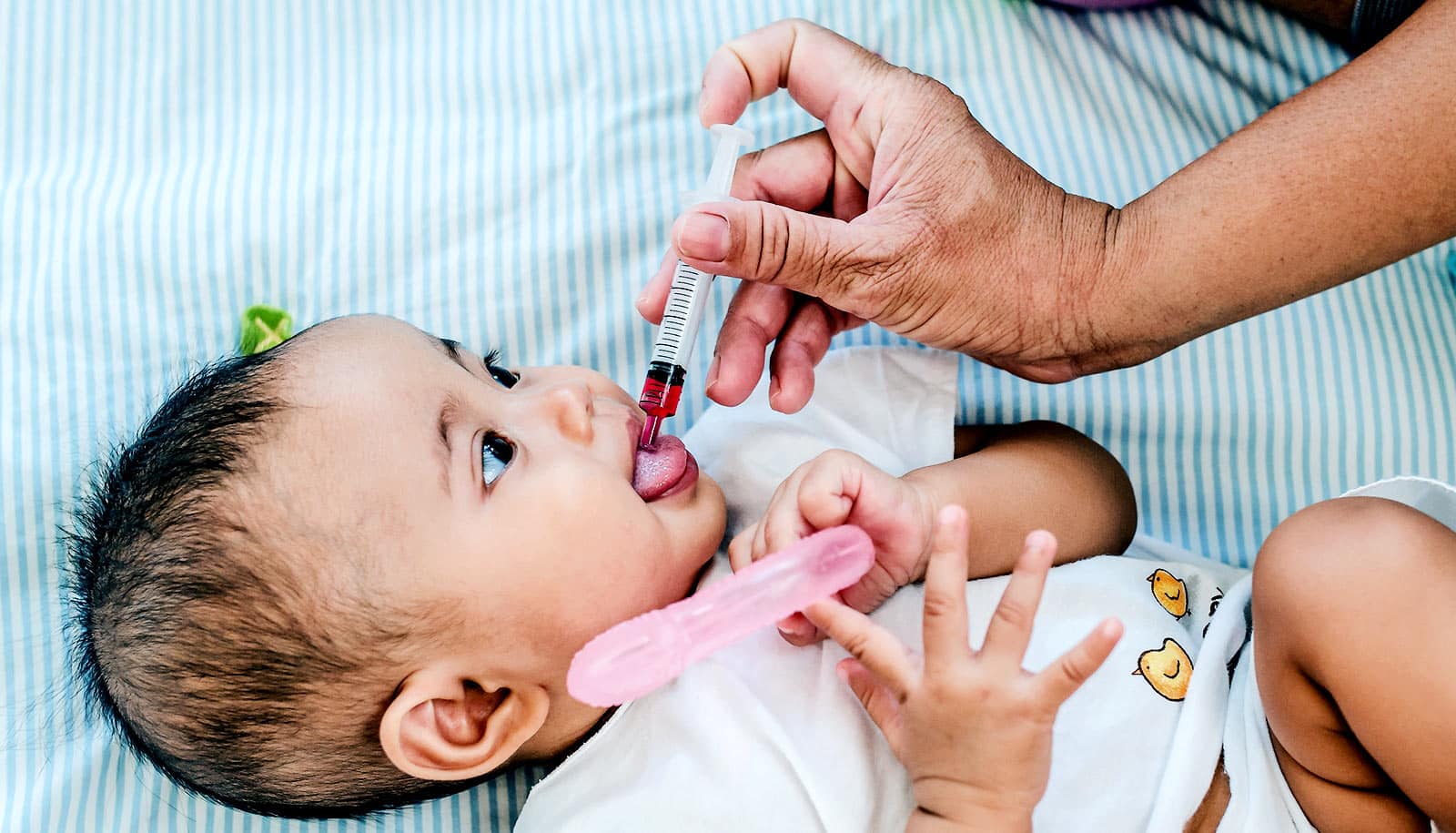
In children with suspected sinusitis, a nasal swab test can tell whether antibiotics are likely to be effective or not, according to a new study.
“Five million kids in the US get prescribed antibiotics for sinusitis each year,” says Nader Shaikh, a pediatrician and professor of pediatrics and clinical and translational science at the University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh and lead author of the study in JAMA.
“Our study suggests that only half of these kids see an improvement in symptoms with antibiotic use, so by identifying who they are, we could greatly reduce unnecessary antibiotic use.”
Sinusitis, which is an inflammation or swelling of the sinuses, can cause congestion, runny nose, discomfort, and difficulty breathing. Doctors often prescribe antibiotics—which target only bacterial infections—to treat the condition, even though viruses may cause it.
“Sinusitis is one of the most common diseases we see in children, but it’s difficult to diagnose because it’s based on the duration of symptoms: If the child has a runny nose or congestion for more than 10 days, we suspect sinusitis,” Shaikh says. “For an ear infection, we can look inside the ear; for pneumonia, we listen to the lungs. But for sinusitis, we have nothing to go on from a physical exam. That was very unsatisfying to me.”
With the goal of developing a better tool to diagnose bacterial sinusitis, Shaikh and his team enrolled about 500 children with sinusitis symptoms from six centers across the US and randomly assigned them to receive either a course of antibiotics or placebo. The researchers also took swabs from inside the nose—much like a COVID-19 test—from each child and tested for the three main types of bacteria involved in sinusitis.
Kids who tested positive for the bacteria had better resolution of symptoms with antibiotic treatment compared to those who did not have bacteria. These findings suggest that testing for bacteria could be a simple and effective way to detect children who are likely to benefit from antibiotics and avoid prescribing antibiotics to those who wouldn’t.
“If antibiotics aren’t necessary, then why use them?” says Shaikh. “These medications can have side effects, such as diarrhea, and alter the microbiome, which we still don’t understand the long-term implications of. Overuse of antibiotics can also encourage antibiotic resistance, which is an important public health threat.”
According to Shaikh, a common belief among parents and doctors is that yellow or green nasal mucus signals a bacterial infection. Although several small studies have suggested that nasal discharge color is not meaningful, Shaikh and his team formally tested this idea by asking parents to identify the hue of their child’s mucus on a color card.
“If kids with green or yellow discharge benefited more from antibiotics than those with clear-colored discharge, we would know that color is relevant for bacterial infection,” says Shaikh. “But we found no difference, which means that color should not be used to guide medical decisions.”
The researchers are now looking at how to best roll out nasal testing in the clinic. A major challenge is that bacterial culture-based tests used in the study are not easy for most family doctors to order and can take several days to get results. A more practical approach could be commercially available molecular testing, which could return results overnight, says Shaikh.
Another possibility could be development of rapid antigen tests that work like COVID-19 at-home testing kits. The researchers also plan to delve deeper into the data from this study to see whether there could be another type of biomarker in nasal discharge indicating the presence of bacteria that would be easier to test for.
Additional coauthors are from Kentucky Pediatric/Adult Research, the University of Wisconsin, and the University of Pittsburgh.
The National Institute of Allergy and Infectious Diseases supported the work.
Source: University of Pittsburgh