New research explains why tail regeneration is perfect in salamanders and imperfect in lizards—and may help clarify why mice can’t regenerate their tails at all.
Stem cells in the spinal cord are the ultimate limiting factor, scientists report in the Proceedings of the National Academy of Sciences.
“The traditional animal model for regeneration is the salamander,” says senior author Thomas P. Lozito, assistant professor in the orthopaedic surgery department, the Center for Cellular and Molecular Engineering, and the McGowan Institute for Regenerative Medicine at the University of Pittsburgh.
“Salamanders can regenerate a wide variety of tissues—brain, heart, parts of their eyes, limbs, tails—but they have whole classes of molecule types and tissues that just aren’t found in mammals, so we really haven’t been able to apply very much of what we found in the salamander to humans.”
“You can easily tell a lizard with a regenerated tail. It doesn’t get anything right.”
If the goal is to translate regeneration research to non-regenerating species like humans, the lizard is a much better model than the salamander, according to Lozito. Lizards are the closest relative to mammals that can regenerate an appendage, and they have a similar genome and biochemistry.
But lizards cannot regenerate lost limbs at all, and their regenerated tails are much simpler than the originals.
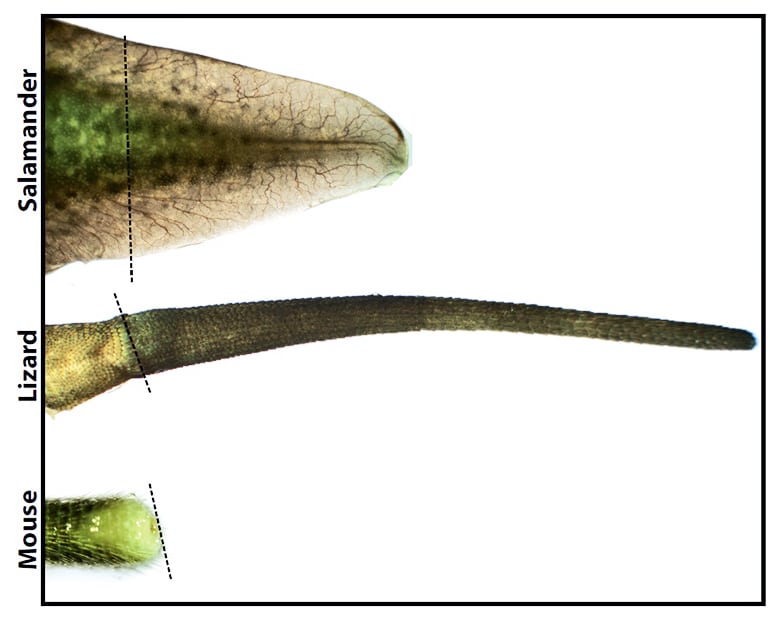
“You can easily tell a lizard with a regenerated tail,” Lozito says. “It doesn’t get anything right. The scales are different; the color pattern is different, and then when you look inside the tail, all the tissues are different. There’s no bone; the skeleton is completely cartilaginous, just tubes within tubes.”
Understanding what separates perfect regeneration in the salamander from imperfect regeneration in the lizard lays the groundwork for bridging the gap to non-regenerating species, he says.
Lozito’s lizard of choice is the mourning gecko, which has several interesting properties, including a high tolerance for transplantation.
“The spinal cord is the master regulator of tail regeneration…”
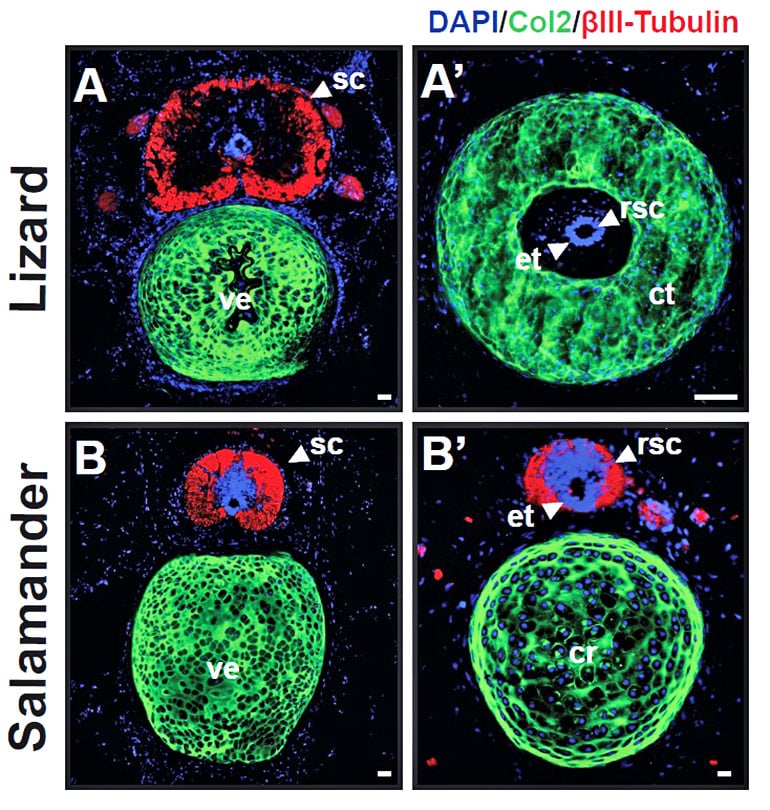
This feature allowed Lozito and colleagues to take neural stem cells—the nascent precursors of neurons and glia, the non-neuronal cells that surround them—from the salamander and insert them into the lizard’s regenerating tail stump. They wanted to see what holds back tail regeneration in the lizard: the biochemical environment or the lizard’s native stem cells.
The transplanted salamander stem cells retained their ability to differentiate into multiple cell types, including neurons. By contrast, lizard neural stem cells could become only glial cells, which don’t process the messages that direct movement and feeling.
“It was a nice surprise,” says lead author Aaron Sun, a physician-scientist trainee who completed part of his research in Lozito’s lab. “And it goes to show that maybe the regenerative processes are still somewhat conserved.”
Fish retinas heal themselves. Could ours, too?
But perhaps the most surprising observation, Sun says, is that the traditionally described “neural stem cells” driving regeneration in the lizard are not “true” neural stem cells at all. Although they check many of the classic boxes, they fall short of a defining characteristic—the ability to spring forth a diversity of cell types.
That explains why there isn’t perfect tail regeneration in the lizard, Lozito says. The neural stem cells can’t produce the different cell types that would be needed to recreate the asymmetries of the original spinal cord, which in turn stymies the development of bony vertebrae.
“The spinal cord is the master regulator of tail regeneration, and these differences that we’re seeing between lizard and salamander tails are due to differences in stem cell quality,” Lozito says. “It’s all because of the neural stem cells.”
Regeneration could be lurking in our genes
The National Institutes of Health funded this research.
Source: University of Pittsburgh



