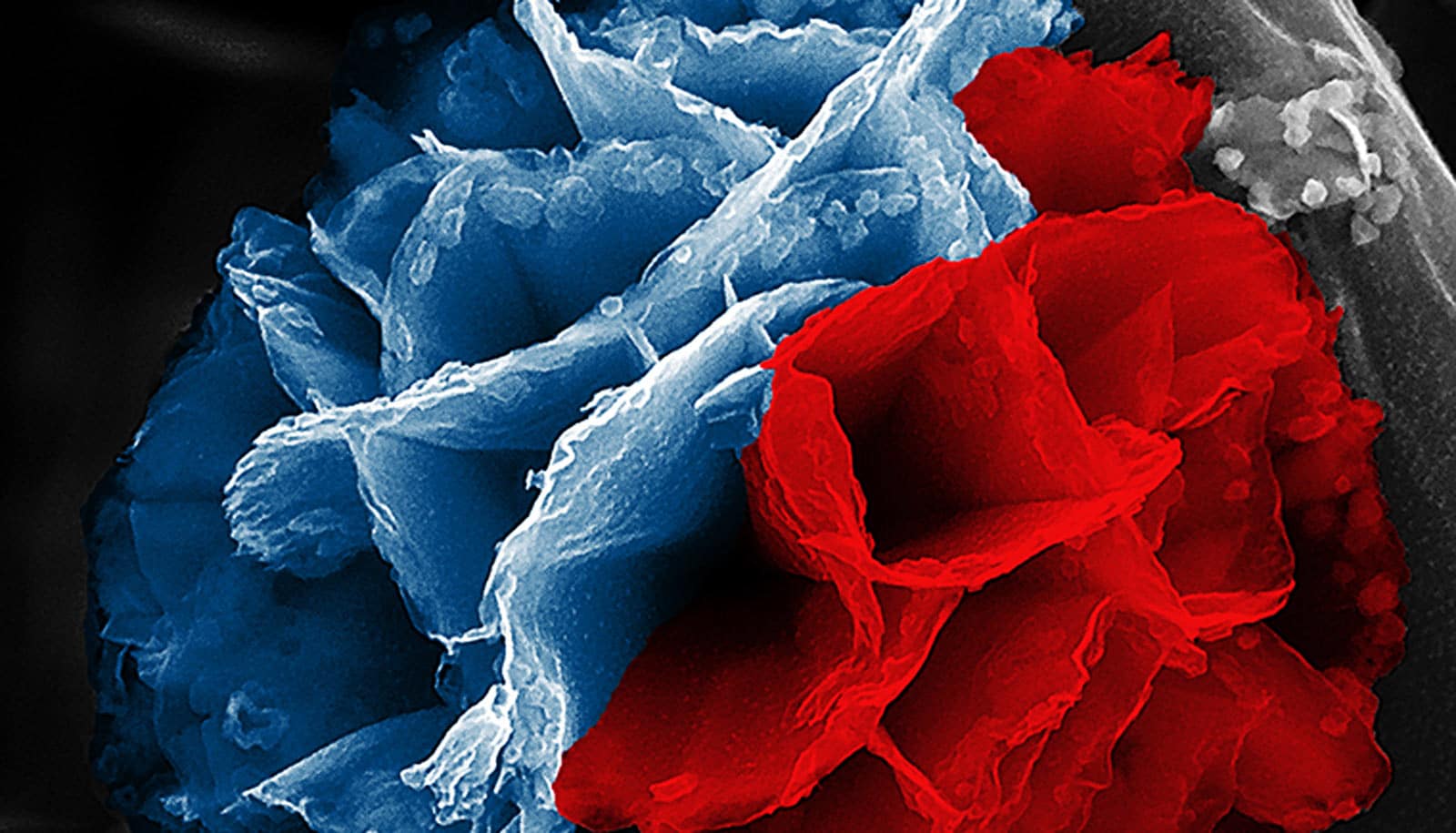
Researchers have developed a straightforward way to make a type of conducting polymers with high surface area—called “nanoflowers”—potentially useful for energy transfer and storage.
If you could brush your cheek against a nanoflower’s microscopic petals, you’d find them cool, hard, and… rusty. Common rust forms the inner skeleton of these lovely and intricate nanostructures, while their outer layer is a kind of plastic.
“Rust will always pose a challenge in Earth’s humid and oxygenated atmosphere,” says Julio M. D’Arcy, assistant professor of chemistry at Washington University in St. Louis and a member of the Institute of Materials Science and Engineering. “Corrosion makes structures fragile and decreases the ability of components to function properly. But in our lab, we’ve learned how to control the growth of rust so that it can serve an important purpose.”
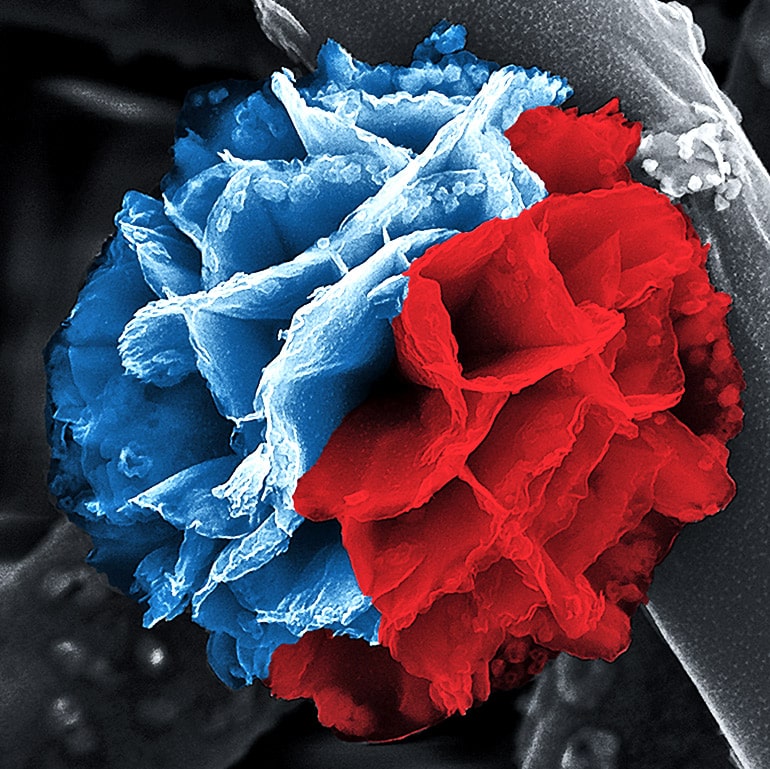
Conducting polymers rely on a combination of organic and inorganic materials—usually a core of metal and a shell of plastic—made in a single batch.
D’Arcy and his team developed a new technique that combines vapor-phase synthesis with solution-based hydrolysis to build three-dimensional nanoflowers, two-dimensional nanoplates, and one-dimensional nanofibers.
This work advances the understanding of the chemical mechanisms involved with depositing the rust and forming the polymer, which will allow scientists to more easily manipulate and engineer the structures of the materials they make.
‘Rusty’ semiconductor circuits can be thinner than silicon
“As chemists, my students and I are fascinated by conducting polymers because we can control their structure during synthesis,” D’Arcy says. “How much electricity the polymers conduct is a function of their chemical pathway and their number of charge carriers, both of which can be optimized during synthesis.”
As for the nanoflowers, D’Arcy says he will be sowing some new seeds soon. There are 16 stable phases of rust, all with different morphologies at the nanoscale—enough for a whole rusted garden.
Researchers report their findings in the journal ACS Applied Nano Materials.