One dose of a new vaccine candidate elicited large increases in respiratory syncytial virus-neutralizing antibodies that continued for several months, researchers report.
The experimental vaccine against RSV, one of the leading causes of infectious disease deaths in infants, has shown promise in the new Phase 1 human clinical trial.
People contract RSV in all stages of life, but it’s most dangerous in the very young and the very old. The virus causes pneumonia, bronchiolitis, and other lower respiratory tract diseases. Every year, RSV sickens millions of people, and more than 100,000 die, mostly in areas that lack access to modern medical care. For infants under one year of age, RSV is second only to malaria for infectious disease deaths.
Scientists have tried to create an RSV vaccine using traditional methods for more than 50 years—and so far, nothing has worked. Instead, Jason McLellan, associate professor of molecular biosciences at the University of Texas at Austin, and colleagues took a new approach, called structure-based vaccine design.
Hear McLellan describe the vaccine’s development:
Shape-shifting protein
Scientists already knew that a certain part of RSV, called the F protein, triggers the human immune system to produce antibodies. But the F protein is a shape shifter—before it infects a cell, it takes one shape and then during infection, it shifts to a second shape. If the immune system encounters an RSV virus with the F protein in the first shape, it makes potent antibodies. But if the protein is in the second shape, it elicits fewer antibodies, and they are not very effective. Producing RSV vaccines using traditional methods usually leads to F proteins in the second shape and a poor antibody response.
This is where the structure-based approach comes in. First, the researchers used a technique called X-ray crystallography to determine the atomic-level structure of the F protein in the first shape. Next, they re-engineered the F protein to take away its shape-shifting ability, locking it in the shape that elicits the best antibodies.
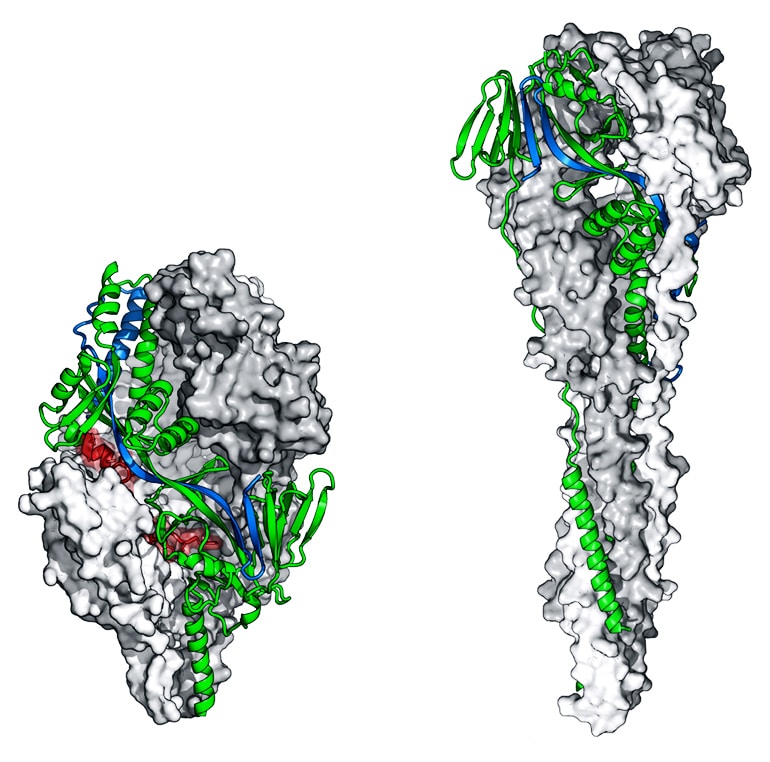
In 2013, they tested several versions as a vaccine in both mice and nonhuman primates. These protein variants elicited high levels of neutralizing antibodies and protected the animals against RSV infection.
“Our first time testing these stabilized molecules in animals, the response was 10-fold higher than anything anyone had ever seen before,” McLellan says. “And at that point, we’re thinking, ‘This is it. We’ve got it.’ That was exciting.”
Next steps for the RSV vaccine
The researchers selected the most promising of these vaccine candidates, DS-Cav1, for clinical evaluation and the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center subsequently manufactured it.
The new report is an interim analysis of data from the first 40 healthy adult volunteers enrolled in the trial, which began in the National Institutes of Health Clinical Center in 2017. Researchers found that the vaccine candidate elicits a greater than 10-fold increase in RSV-neutralizing antibodies, compared with the number of antibodies a person produces naturally from RSV exposure earlier in life.
The results are promising, but McLellan is careful to put them in perspective.
“The Phase 1 just asks: Is it safe and is it eliciting the types of antibodies and response that we were hoping to see?” he says. “It still needs to go through Phase 2 and Phase 3, looking at efficacy such as, is it reducing the severity of disease, or is it reducing hospitalizations?”
Many drugs fail to make it all the way through clinical trials. But if this one does, or another based on the same F protein structure that he helped discover, McLellan says it could be a game changer.
“If it works reasonably well and we prevent 70 to 80% of all deaths, just think of all the little infants and toddlers we’d save,” McLellan says. “There aren’t that many vaccines in the world, and so if we’re able to actually participate in making one that works and saves lives, that would be awesome.”
The research appears in Science.
Additional information about the Phase 1 trial of DS-Cav1 (also known as VRC 317) is available at clinicaltrials.gov with the trial identifier NCT03049488. Final results of the trial are expected next year.
McLellan has submitted required financial disclosure forms with the University of Texas at Austin. McLellan is an inventor on several patent applications related to this research that the National Institutes of Health filed, from which he is receiving royalties. Additional researchers from the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center contributed to the work.
Source: Texas A&M University