Perpetually hungry, microscopic worms will spend up to 20 minutes seeking out snacks in their immediate surroundings before looking elsewhere. Now, scientists know what’s going on in their brains that drive the behavior.
In a study, which appears in Neuron, researchers describe neural mechanisms responsible for the local search, showing that either smell- or touch-related cues trigger the behavior.
Snack search
Caenorhabditis elegans (C. elegans) feed on bacteria. And when their environment is teeming with edible microbes, they don’t have to move much to remain sated.
But when food availability decreases, the worms start wiggling around a bit. First, they extensively search the area in which they last encountered sustenance, circling a small region for 10 to 20 minutes. Eventually, they widen their search perimeter—a strategy that resembles, and is possibly related to—some aspects of human behavior.

“If you lose your keys, you first search locally: in your wallet, then in your coat, and other nearby places. And you do this repeatedly,” says Cori Bargmann, professor at Rockefeller University. “But if that doesn’t work, at some point you begin searching a broader area.”
Hungry insects, reptiles, fish, and mammals all make the switch from a local to global search, suggesting that it may represent a conserved foraging strategy. With a uniquely small and well-characterized nervous system, C. elegans provided Bargmann and colleagues with handy tools to study the basic brain mechanisms driving the behavior.
Local or global?
To identify cellular components involved in local search, biomedical fellow Alejandro López-Cruz first analyzed the genes of C. elegans mutants that failed to produce this behavior—worms that skipped the whole “local” phase and proceeded directly to global search.
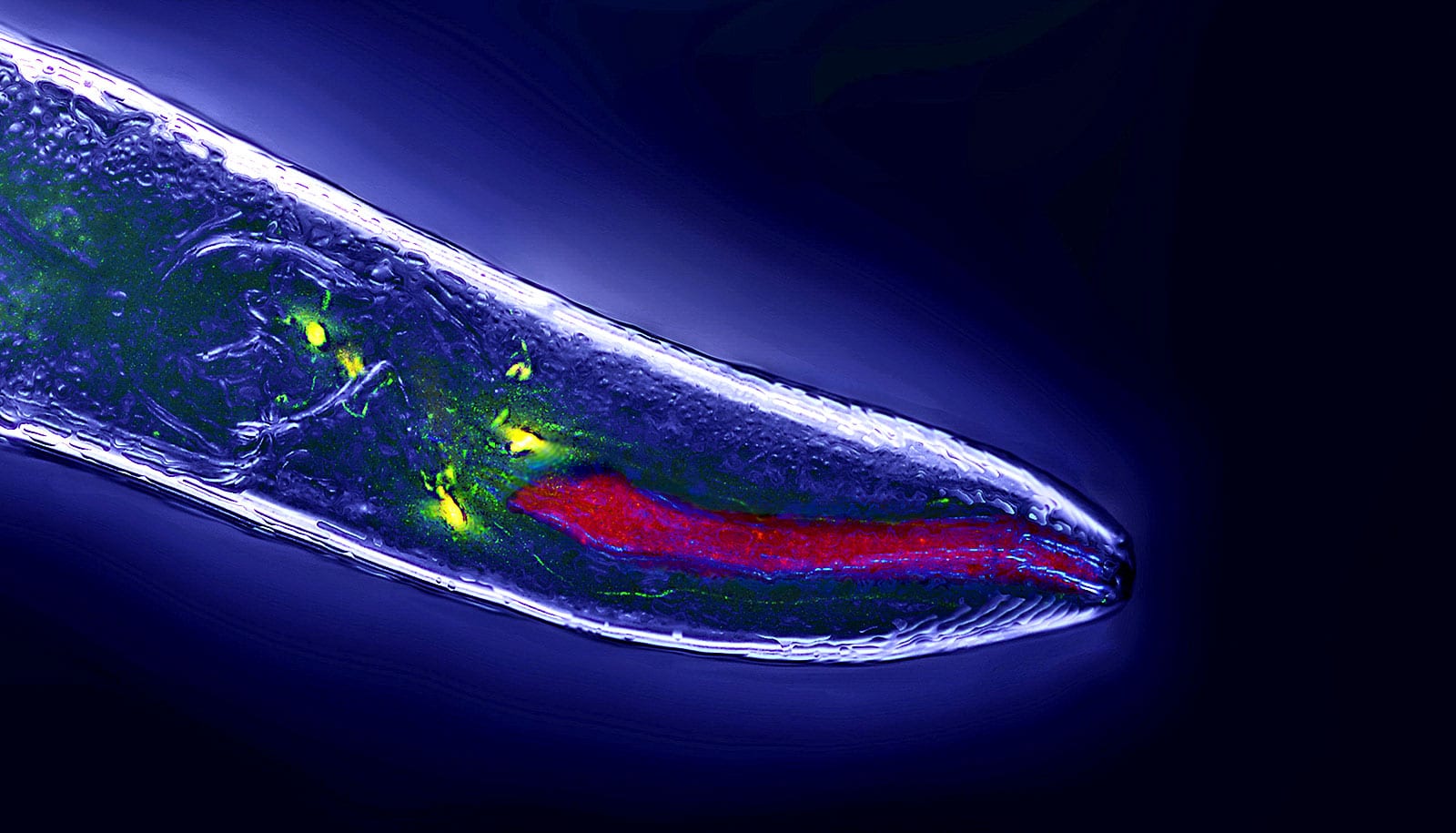
He discovered these animals have a defective form of a protein known as MGL-1, a receptor for the neurochemical glutamate. Additional experiments found MGL-1 receptors on two C. elegans neurons involved in detecting food: one called AIA, which receives input from chemosensory cells sensitive to food odors; and another called ADE, which connects to mechanosensory cells responsive to food texture.
Mechanosensory neurons and ADE, the researchers found, don’t physically connect to chemosensory neurons and AIA—suggesting that smell and touch responses function independently. Supporting these findings, the researchers showed that animals using only one sensory system can effectively detect and react to food removal.
“If you knock out glutamate from chemosensory and mechanosensory cells then you lose the behavior. But if you knock out only one, the animals respond normally,” says López-Cruz. “This tells us that there are two parallel and redundant pathways for detecting food.”
“That makes sense,” adds Bargmann, “because food is probably too important to survival to represent it in just a single form.”
Missing meals
Investigating the longevity of local search, the researchers found that when mechanosensory or chemosensory neurons are stimulated, they act on MGL-1 receptors to modify the activity of ADE or AIA cells for minutes at a time. This cellular modification, in turn, triggers other neurons responsible for local search.
In short: When food goes missing, worm brain activity changes for an extended period of time, yielding an extended search period. This process, says López-Cruz, seems to represent the formation of a memory related to changes in food availability.
“When we take the worm’s food away, we are essentially giving it a food-removal stimulus. The behavioral change that follows outlasts that stimulus by fifteen minutes, which is consistent with short-term memory,” he says.
The switch from local to global search, says Bargmann, seems to reflect the process of forgetting.
“A worm’s memory defines the period of local search,” she says. “And when it forgets, the animal moves on to longer-range exploration.”
Source: Rockefeller University