A new imaging device could detect a biomarker of Alzheimer’s disease in the retina, research in mice indicates.
The device can measure both the thickness and texture of the various layers of the retina at the back of the eye, potentially offering a widespread early warning system for the disease.
“Previous research has seen a thinning of the retina in Alzheimer’s patients, but by adding a light-scattering technique to the measurement, we’ve found that the retinal nerve fiber layer is also rougher and more disordered,” says Adam Wax, professor of biomedical engineering at Duke University.
“Our hope is that we can use this insight to create an easy and cheap screening device that wouldn’t only be available at your doctor’s office, but at places like your local pharmacy as well.”
Currently, doctors can only diagnose Alzheimer’s disease after a patient begins to show symptoms of cognitive decline. Even then, the only way to definitively determine that Alzheimer’s was the cause is with expensive MRI and PET scans or through an autopsy.
But if early interventions such as drugs and mental exercise can halt disease progress, patients can have a greatly improved quality of life. This is why researchers look for biomarkers they can use as early warning signs of the disease.
Alzheimer’s biomarker in retina layers
One such potential biomarker comes from the retina, which is literally an extension of the brain and part of the central nervous system. Previous research has shown that Alzheimer’s can cause structural changes to the retina, most notably a thinning of the inner retinal layers.
“The retina can provide easy access to the brain, and its thinning can be indicative of a decrease in the amount of neural tissue, which can mean that Alzheimer’s is present,” says Wax.
Other diseases such as glaucoma and Parkinson’s disease, however, can also cause a thinning of the retina. Inconsistent test results might also come from differences between the machines most often used for these types of measurements, optical coherence tomography (OCT) devices, and how researchers use them.
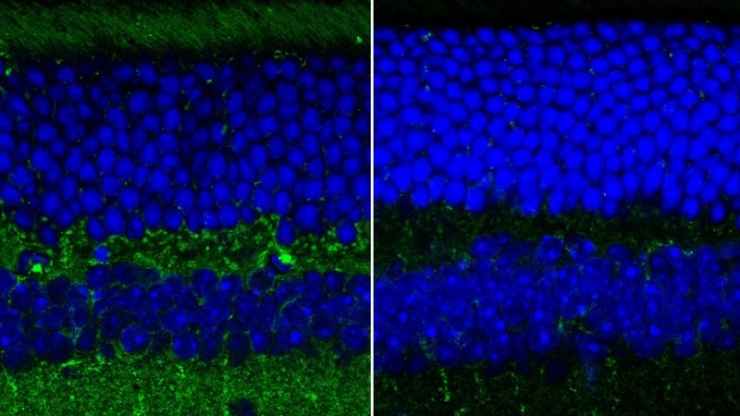
In the new paper, Wax and graduate student Ge Song show that the topmost layer of neurons in the retina of a mouse model of Alzheimer’s disease exhibit a change in their structural texture. Combined with data on the changes in thickness of this layer, the new measurement could prove to be a more easily accessible biomarker of Alzheimer’s.
“Our new approach can measure the roughness or texture of the nerve fiber layer of the inner retina,” Song says. “It can provide a quick and direct way to measure structural changes caused by Alzheimer’s, which has great potential as a biomarker of the disease.”
Low-cost testing
OCT, the optical analog of ultrasound, sends waves of light into tissues and measures how long they take to come back. While it is an extremely useful imaging technique commonly used to make a wide array of diagnoses, it has limitations.
To gather more data, Wax and Song added a measurement called angle-resolved low-coherence interferometry (a/LCI), which uses the angles of the scattered light to gather more information about the tissue’s structure. By combining the two measurements, the researchers can extract both thickness and structural information about each layer of the retina.
“The a/LCI measurements complement the thickness measurements to improve the potential utility of more quantitative biomarkers for Alzheimer’s,” Song says. “You can’t get textural and structural information about the retina with OCT alone. You need both imaging modalities. That’s the key innovation.”
The researchers are now working to incorporate this added ability into a low-cost OCT system that Wax is developing through a spinoff company called Lumedica. While traditional OCT machines weigh more than 60 pounds, take up an entire desk, and cost anywhere between $50,000 and $120,000, Wax’s design weighs four pounds, is about the size of a lunch box, and, Wax expects, could cost less than $15,000.
The key to Wax’s design is a 3D-printed part that uses symmetry to compensate for mechanical inconsistencies that can arise in traditional OCT devices because of things as small as a subtle shift in temperature. Song also is working on a 3D-printed rotational prism, allowing the a/LCI to scan the entire retina.
“We’re excited because this research shows a new way of using low-cost OCT technologies outside of simply screening for traditional retinal diseases,” Wax says. “If we can use these devices as a window into early signs of neurodegenerative diseases, maybe we can help people get into an early intervention treatment program before it’s too late.”
The study appears in Scientific Reports. The National Institutes of Health supported the work.
Source: Duke University