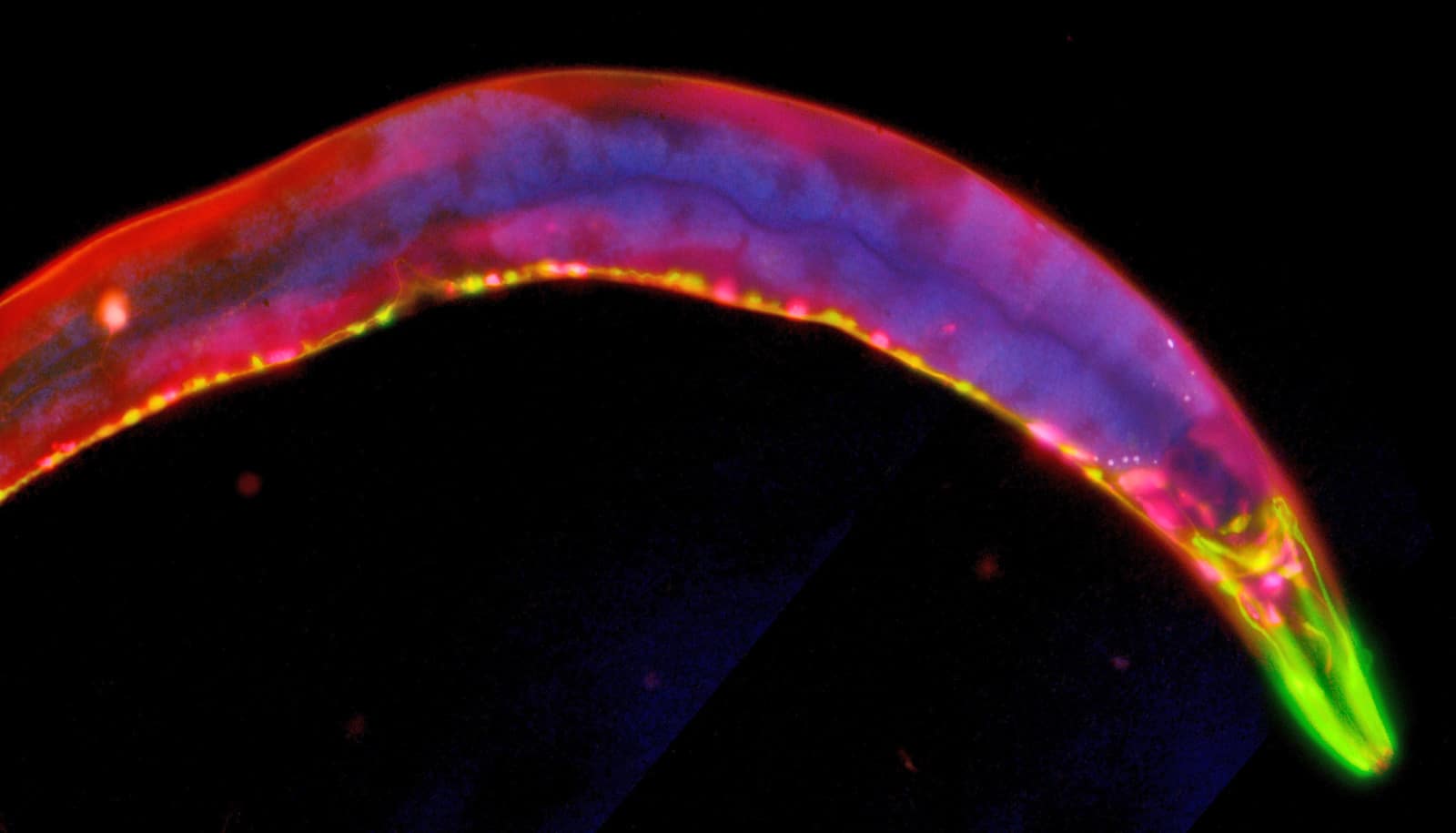
A newly discovered gene in the roundworm C. elegans could be the “missing link” that determines when it’s time for puberty to begin, report researchers.
Two genes, LIN28 and MKRN3, are associated with precocious puberty in humans, in which children as young as six may start developing adult features. These genes are in all animals, including C. elegans, in which they also control the juvenile-to-adult transition. Until the new discovery, it was unclear how these two genes are connected.
The more obvious signs of the transition of juvenile-to-adult tend to be external—body morphology, matured genitalia—but nervous system changes also happen at the same time. In humans, the maturation of the brain during adolescence is associated with increased vulnerability to a variety of neuropsychiatric disorders, so a better understanding of these processes is important for understanding mental health as well as basic neurobiology.
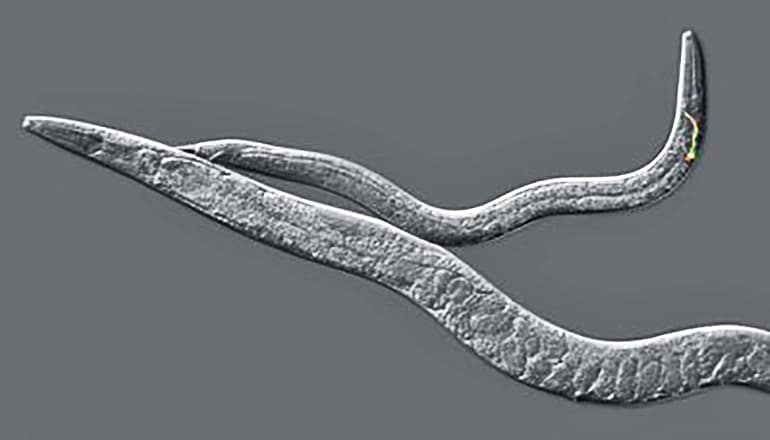
Two new studies in Developmental Cell and eLife identify a new developmental timing mechanism involving a long non-coding RNA in the microscopic roundworm C. elegans. The research reveals a surprising new molecular mechanism that controls the timing of sex-specific changes in body shape, the maturation of neural circuits, and behavior.
Model organism
Researchers have long used C. elegans to understand fundamental mechanisms in biology. Many of the discoveries researchers make using these worms apply throughout the animal kingdom and this research has led to a broader understanding of human biology. In fact, three Nobel Prizes in medicine and chemistry have been awarded for discoveries involving C. elegans.
The researchers identified a new gene that, when disrupted, delays the transition from the juvenile to the adult stage. Surprisingly, this gene, called lep-5, does not act as a protein, as most genes do. Instead, it functions as a long non-coding RNA (lncRNA), a recently discovered class of genes whose functions remain largely mysterious.
The team observed that this lncRNA is important for promoting the juvenile-to-adult transition by directly interacting with LIN-28 and LEP-2, a C. elegans gene similar to MKRN3. Because the human versions of LEP-2 and LIN-28 are both involved in the timing of puberty, the new research suggests that a yet-to-be-discovered lncRNA might be essential to this process in humans as well.
Roundworm puberty
In the roundworm nervous system, some neural circuits undergo a functional transition in males as they become sexually mature adults, which is critical for generating adult-specific behaviors important for reproductive success. The male tail also undergoes a change in shape that enables mating behavior.
The researchers found that this same pathway controls both the functional maturation of these circuits and the shape of the tail. Roundworms carrying mutations in lep-5 become physically mature adults, but their nervous system remains arrested in the juvenile stage, and their tails retain a juvenile form.
With respect to changes in behavior, the pathway regulates this timing by acting in the nervous system itself, not in a tissue that sends timing signals to the nervous system. Moreover, individual neurons manage their own developmental clocks. A timed “pulse” of lep-5 activity during the juvenile stage causes LIN-28 to become inactive, allowing the transition to adulthood to proceed.
Continued studies of the mechanisms identified in these studies will help scientists better understand the ways in which genetic and environmental cues regulate the transition to adulthood in humans.
The National Institute of General Medical Sciences and National Science Foundation supported the research. Additional researchers from the University of Rochester and New York University contributed to the study.
Source: University of Rochester