Most ovarian cancers may have their genetic origins in the fallopian tubes, the thin fibrous tunnels that connect the ovaries to the uterus, a small study suggests.
The study, providing new evidence for a link that some scientists have suspected, could eventually lead to new ways to prevent or treat the disease.
Ovarian cancer, the fifth-largest cause of cancer deaths in women, is diagnosed late in most patients; fewer than 30 percent of women with the disease survive beyond 10 years.
“Ovarian cancer treatments have not changed much in many decades, and this may be, in part, because we have been studying the wrong tissue of origin for these cancers,” says Victor Velculescu, leader of the study in Nature Communications.
“If studies in larger groups of women confirm our finding that the fallopian tubes are the site of origin of most ovarian cancer, then this could result in a major change in the way we manage this disease for patients at risk,” says Velculescu, professor of oncology at the Johns Hopkins University’s Kimmel Cancer Center.
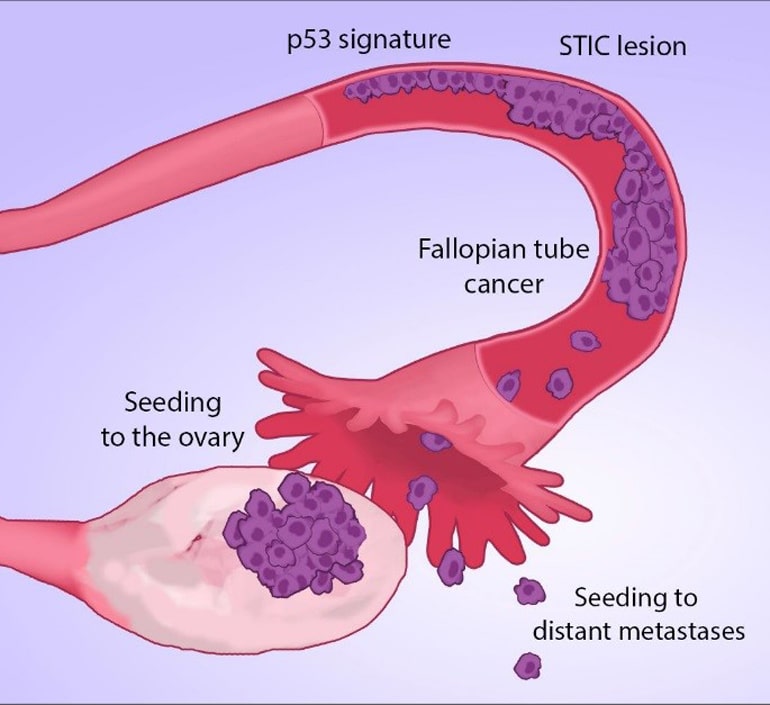
STIC lesions
Scientists at Johns Hopkins and the Dana Farber Cancer Institute collected tissue samples from five women diagnosed with high-grade serous ovarian tumors, which account for three-quarters of the estimated 22,000 women diagnosed with ovarian cancer each year in the United States. The samples included normal cells, ovarian cancers, metastases that had spread elsewhere, and small cancers found in the fallopian tubes, which included single-cell layers of cancer called “p53 signatures” and serous tubal intraepithelial carcinoma, or STIC lesions.
The scientists also collected samples from STIC lesions and normal cells from four women who had had their ovaries and fallopian tubes removed for preventive reasons. Three had the procedure because they carried hereditary gene mutations in the BRCA gene, linked to ovarian and breast cancer. The other had a pelvic mass.
Because some of the cancers were extremely small, researchers developed a way to isolate the relatively few cancer cells from the larger mass of adjacent normal cells. They then searched for mistakes in the cancer cell DNA sequences. They looked both for areas where just one DNA molecule was switched for another and for spots where large regions of DNA in a particular chromosome were altered.
All nine patients had lost identical regions of chromosome 17, where the cancer-linked p53 gene is located, in each of the cancer samples, including the early stage STIC lesions. That suggested that the “misprinted” or flawed p53 gene is an early step in ovarian cancer development.
Why ovarian cancer is so good at beating chemo
All nine patients also had lost portions of chromosomes containing one or both BRCA1 and BRCA2 genes, which have long been linked to hereditary as well as sporadic breast and ovarian cancers. Four patients had deletions in chromosome 10 where another cancer-linked gene called PTEN is located.
Reasoning that there would be fewer mutations in the original cancer cells than in their successors, the scientists created an evolutionary tree among the ovarian cancers in the five women. They concluded that each of the women’s cancers began with mistakes in STIC or earlier lesions located in the fallopian tubes. That suggests that the development of cancer in the ovaries is the result of a seeding event from an initial tumor in the fallopian tubes that already contains key DNA changes.
Better screening
The results also showed that that ovarian cancer developed from STIC lesions in an average of 6.5 years. When the cancers reached patients’ ovaries, however, the progression to metastatic disease was rapid, within an estimated two years on average.
Can these 16 compounds flag ovarian cancer sooner?
“This aligns with what we see in the clinic, that newly diagnosed ovarian cancer patients most often already have widespread disease,” Velculescu says.
“The window of time that exists between the development of a STIC lesion and metastatic disease highlights the importance of new screening approaches, such as liquid biopsy methods, for early detection of ovarian cancer,” he adds.
Velculescu notes that the fallopian-first theory may not apply to less-common types of ovarian cancer.
Others who worked on the project come from Johns Hopkins, Dana-Farber, Brigham and Women’s Hospital and Harvard Medical School, the University of Pennsylvania, Geneva University Hospital, Seirei Mikatahara Hospital, and Personal Genome Diagnostics.
The Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, Commonwealth Foundation, National Institutes of Health, Defense Department, Honorable Tina Brozman Foundation for Ovarian Cancer Research, SU2C-DCS International Translational Cancer Research Dream Team Grant, Foundation for Women’s Wellness, and Richard W. TeLinde Gynecologic Pathology Laboratory Endowment all funded the research.
Velculescu is a founder of Personal Genome Diagnostics and a member of its scientific advisory board and board of directors. Velculescu owns Personal Genome Diagnostics stock, which is subject to certain restrictions. Johns Hopkins manages the terms in accordance with its conflict of interest policies.
Source: Johns Hopkins University