High levels of human-made CO2 causes ocean acidification that is rapidly dissolving the seafloor, a new study warns.
Normally the deep sea bottom is a chalky white. It’s composed, to a large extent, of the mineral calcite (CaCO3) formed from the skeletons and shells of many planktonic organisms and corals.
The seafloor plays a crucial role in controlling the degree of ocean acidification. The dissolution of calcite neutralizes the acidity of the CO2, and in the process prevents seawater from becoming too acidic.
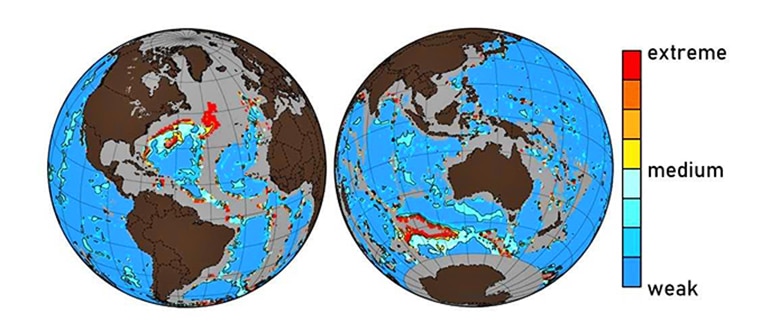
But these days, at least in certain hotspots such as the northern Atlantic and the southern oceans, the ocean’s chalky bed is becoming more of a murky brown. Because of human activity, the level of CO2 in the water so high—and the water is so acidic—that the calcite is simply dissolving.
Fated future
Researchers say they believe what they are seeing today is only a preview of the way that the ocean floor will most likely be affected in future.
“Because it takes decades or even centuries for CO2 to drop down to the bottom of the ocean, almost all the CO2 created through human activity is still at the surface,” says lead author Olivier Sulpis who is working on his PhD in the earth and planetary sciences department at McGill University.
“But in the future, it will invade the deep-ocean, spread above the ocean floor, and cause even more calcite particles at the seafloor to dissolve,” Sulpis says.
“The rate at which CO2 is currently being emitted into the atmosphere is exceptionally high in Earth’s history, faster than at any period since at least the extinction of the dinosaurs. And at a much faster rate than the natural mechanisms in the ocean can deal with, so it raises worries about the levels of ocean acidification in future,” he explains.
Ocean in the lab
Because it is difficult and expensive to obtain measurements in the deep-sea, the researchers created a set of seafloor-like microenvironments in the laboratory, reproducing abyssal bottom currents, seawater temperature and chemistry, and sediment compositions.
The experiments helped them understand what controls the dissolution of calcite in marine sediments and allowed them to quantify precisely its dissolution rate as a function of various environmental variables. By comparing pre-industrial and modern seafloor dissolution rates, they were able to extract the anthropogenic fraction of the total dissolution rates.
“Just as climate change isn’t just about polar bears, ocean acidification isn’t just about coral reefs.”
The speed estimates for ocean-bottom currents came from a high-resolution ocean model developed by Brian Arbic, a physical oceanographer and associate professor at the University of Michigan, and a former postdoctoral fellow in his laboratory, David Trossman, who is now a research associate at the University of Texas-Austin.
“When David and I developed these simulations, applications to the dissolution of geological material at the bottom of the oceans were far from our minds,” Arbic says. “It just goes to show you that scientific research can sometimes take unexpected detours and pay unexpected dividends.”
“Just as climate change isn’t just about polar bears, ocean acidification isn’t just about coral reefs,” Trossman adds. “Our study shows that the effects of human activities have become evident all the way down to the seafloor in many regions, and the resulting increased acidification in these regions may impact our ability to understand Earth’s climate history.”
“This study shows that human activities are dissolving the geological record at the bottom of the ocean,” says Arbic. “This is important because the geological record provides evidence for natural and anthropogenic changes.”
In future work, the researchers plan to look at how this deep ocean bed dissolution is likely to evolve over the coming centuries, under various potential future CO2 emission scenarios. They believe that it is critical for scientists and policy makers to develop accurate estimates of how acidification humans cause will affect marine ecosystems over the long-term.
The Natural Sciences and Engineering Research Council of Canada (NSERC) and the US National Science Foundation funded the work, which appears in the Proceedings of the National Academy of Sciences.
Source: McGill University



