When researchers transplanted specific human brain cells into animal models of multiple sclerosis and other white matter diseases, the cells repaired damage and restored function, according to a new study.
The study provides one of the final pieces of scientific evidence necessary to advance this treatment strategy to clinical trials.
“These findings demonstrate that through the transplantation of human glial cells, we can effectively achieve remyelination in the adult brain,” says lead author Steve Goldman, professor of neurology and neuroscience at the University of Rochester Medical Center and co-director of the Center for Translational Neuromedicine.
“These findings have significant therapeutics implications and represent a proof-of-concept for future clinical trials for multiple sclerosis and potential other neurodegenerative diseases.”
As reported in Cell Reports, the findings represent the culmination of more than 15 years of research understanding support cells found in the brain called glia, how the cells develop and function, and their role in neurological disorders.
Goldman’s lab has developed techniques to manipulate the chemical signaling of embryonic and induced pluripotent stem cells to create glia. A subtype of these, called glial progenitor cells, gives rise to the brain’s main support cells, astrocytes and oligodendrocytes, which play important roles in the health and signaling function of nerve cells.
In multiple sclerosis, an autoimmune disorder, glial cells are lost during the course of the disease. Specifically, the immune system attacks oligodendrocytes. These cells make a substance called myelin, which, in turn, produce the “insulation” that allows neighboring nerve cells to communicate with one another.
As myelin is lost during disease, signals between nerve cells become disrupted, which results in the loss of function reflected in the sensory, motor, and cognitive deficits.
In the early stages of the disease, referred to as relapsing multiple sclerosis, oligodendrocytes replenish the lost myelin. However, over time these cells become exhausted, can no longer serve this function, and the disease becomes progressive and irreversible.
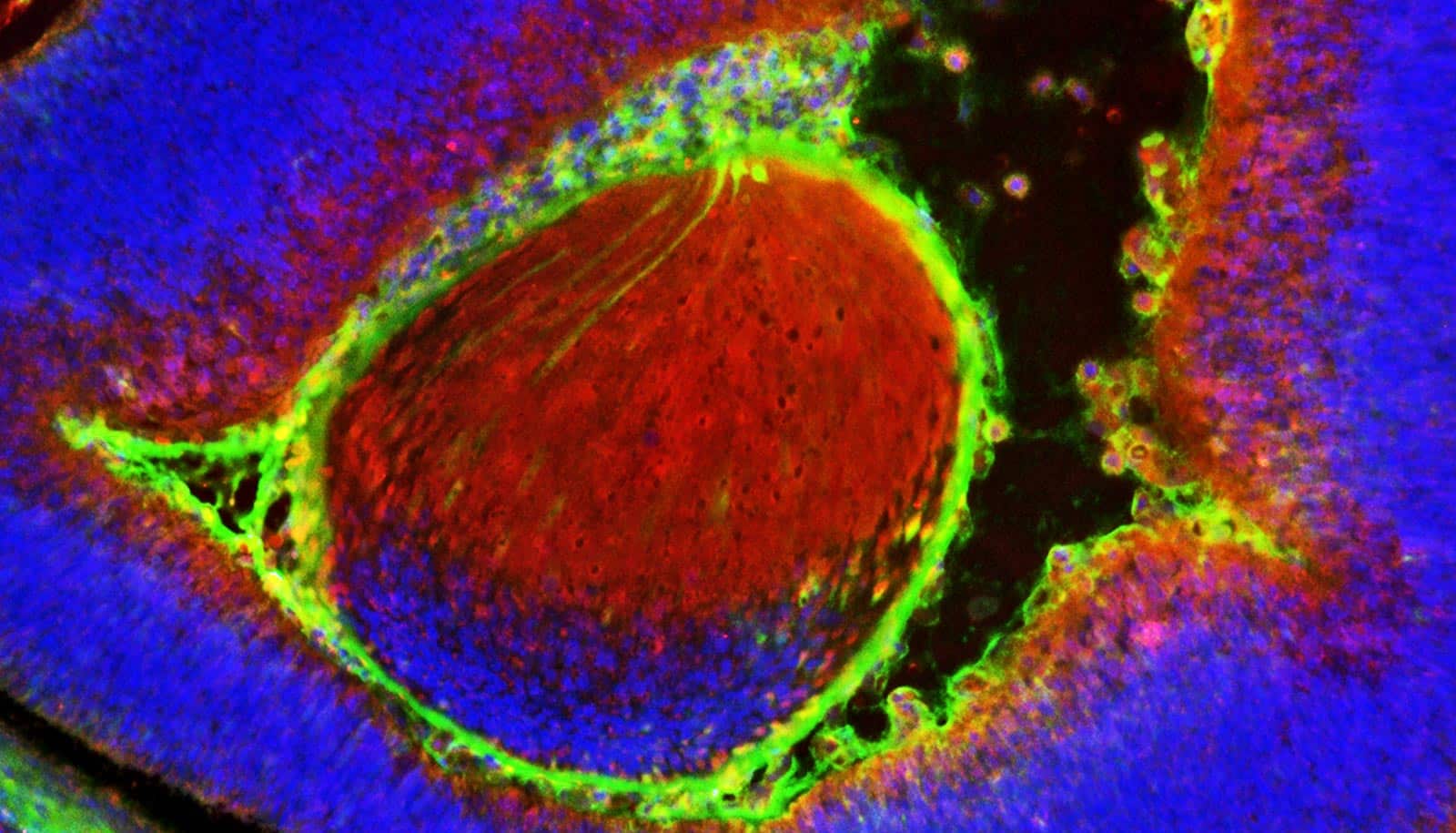
In the new study, Goldman’s lab showed that when researchers transplanted human glia progenitor cells into adult mouse models of progressive multiple sclerosis, the cells migrated to where the brain needed them, created new oligodendrocytes, and replaced the lost myelin.
The study also shows that this process of remyelination restored motor function in the mice. The researchers believe this approach could also apply to other neurological disorders, such as pediatric leukodystrophies—childhood hereditary diseases in which myelin fails to develop—and certain types of stroke affecting the white matter in adults.
A University of Rochester start-up company Oscine Therapeutics, is in the process of developing the research. The company’s experimental transplant therapy for multiple sclerosis and other glial diseases, such as Huntington’s disease, is currently under early FDA review for clinical trials. Goldman is the scientific founder, an officer, and holds equity in the company.
The National Institute of Neurological Disorders and Stroke, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Mathers Charitable Foundation, the New York Stem Cell Research Program, the Oscine Corporation, and Sana Biotechnology funded the work.
Goldman also holds an appointment at the University of Copenhagen. The Novo Nordisk Foundation and the Lundbeck Foundation supports his work there.
Source: University of Rochester