The sinking carcasses of fish from near-surface waters deliver toxic mercury pollution to the most remote and inaccessible parts of the world’s oceans, research finds. That includes the 36,000-foot-deep Mariana Trench in the northwest Pacific.
The research also indicates that most of that mercury began its long journey to the deep-sea trenches as atmospheric emissions from coal-fired power plants, mining operations, cement factories, incinerators, and other human activities.
The research team analyzed the isotopic composition of mercury in fish and crustaceans collected at the bottom of two deep-sea trenches in the Pacific. The team reports its findings in the Proceedings of the National Academy of Sciences.
“Mercury that we believe had once been in the stratosphere is now in the deepest trench on Earth,” says environmental geochemist Joel Blum, lead author of the PNAS paper and a professor in the University of Michigan department of earth and environmental sciences.
“It was widely thought that anthropogenic mercury was mainly restricted to the upper 1,000 meters of the oceans, but we found that while some of the mercury in these deep-sea trenches has a natural origin, it is likely that most of it comes from human activity.”
At a scientific meeting in June, Blum’s team and a Chinese-led research group independently reported the detection of human-derived mercury in deep-sea-trench organisms.
The Chinese researchers, who reported their findings in Nature Communications, concluded that the mercury gets to the deep-sea trenches by hitching a ride on microscopic particles of sinking organic matter—including fecal material and dead plankton—that constantly rain down from the upper oceans.
But in their PNAS paper, Blum and his colleagues suggest that a more likely explanation is that sinking carrion from fish that feed in the upper ocean delivers most of the mercury to the trenches.
Does this affect seafood?
Why does it matter whether deep-sea-trench mercury came from sinking fish carcasses or from the steady rain of tiny bits of detritus?
Because scientists and policymakers want to know how changing global mercury emissions will affect the levels found in seafood. While mercury emissions have declined in recent years in North America and Europe, China and India continue to expand their use of coal, and global-scale mercury emissions are rising.
To determine the likely impact on seafood, researchers rely on global models. And refining those models requires the clearest possible understanding of how mercury cycles within the oceans and between the oceans and the atmosphere, according to Blum.
“Yes, we eat fish caught in shallower waters, not from deep-sea trenches,” he says. “However, we need to understand the cycling of mercury through the entire ocean to be able to model future changes in the near-surface ocean.”
Mercury is a naturally occurring element, but human activities emit more than 2,000 metric tons of it into the atmosphere each year. This inorganic mercury can travel thousands of miles before being deposited onto land and ocean surfaces, where microorganisms convert some of it to methylmercury, a highly toxic organic form that can accumulate in fish to levels that are harmful to humans and wildlife.
Mercury in the deepest trench
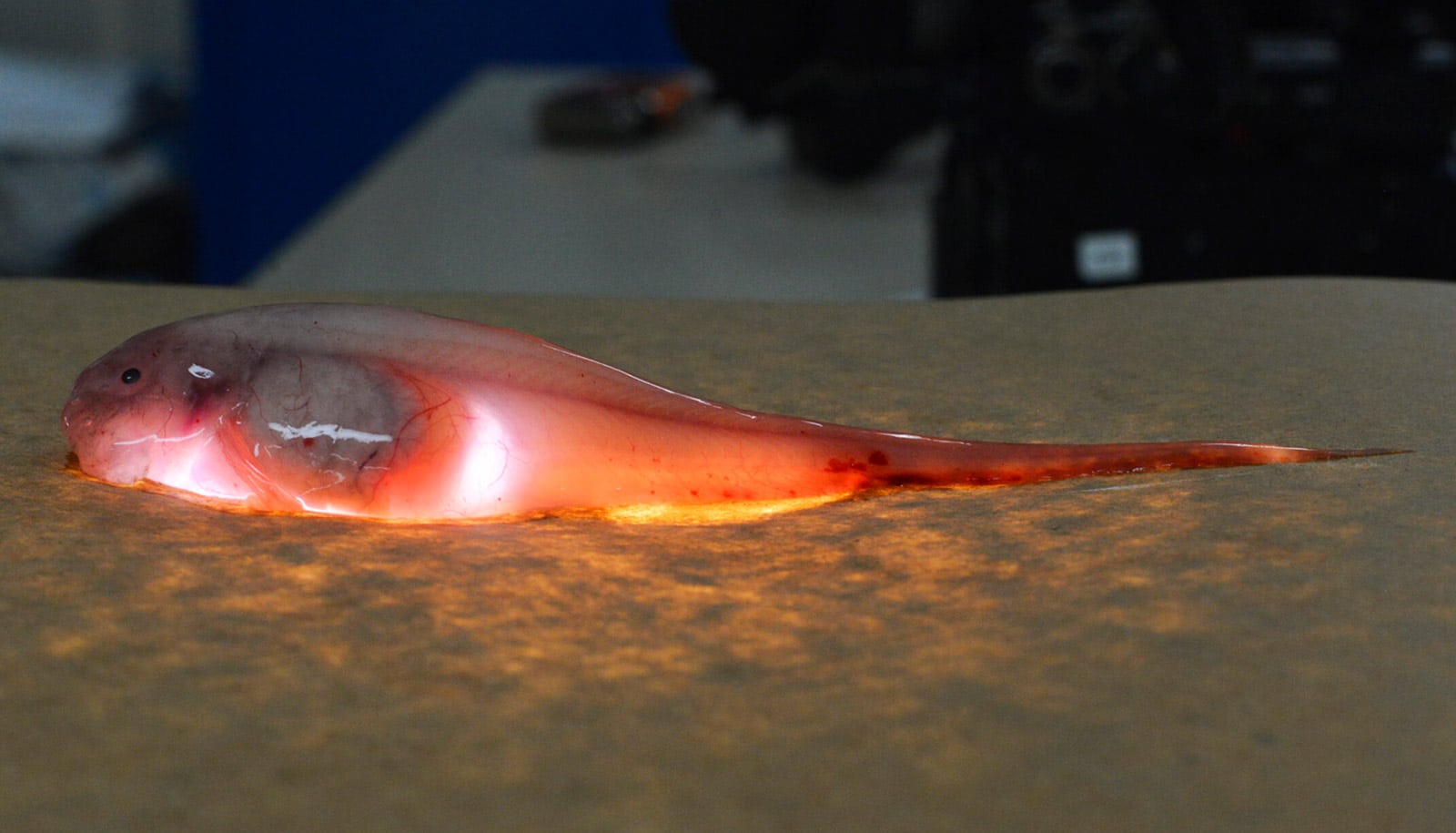
In their study, Blum and his colleagues analyzed the isotopic composition of methylmercury from the tissues of snailfish and crustaceans called amphipods collected at depths of up to 33,630 feet in the Mariana Trench in the northwest Pacific, southwest of Guam. Other samples were collected at depths of up to 32,800 feet in the Kermadec Trench in the southwest Pacific, northeast of New Zealand.
“These samples were challenging to acquire, given the trenches’ great depths and high pressures,” says coauthor Jeffrey Drazen, an oceanographer at the University of Hawaii. “The trenches are some of the least studied ecosystems on Earth, and the Mariana snailfish was only just discovered in 2014.”
Mercury has seven stable (nonradioactive) isotopes, and the ratio of the different isotopes provides a unique chemical signature, or fingerprint, that can be used as a diagnostic tool to compare environmental samples from various locations.
The researchers used these fingerprinting techniques—many of which were developed in Blum’s lab—to determine that the mercury from deep-sea-trench amphipods and snailfish had a chemical signature that matched the mercury from a wide range of fish species in the central Pacific that feed at depths of around 500 meters (1,640 feet). Blum and colleagues analyzed those central Pacific fish during a previous study.
At the same time, they found that the isotopic composition of the mercury in sinking particles of detritus, the delivery mechanism favored by the Chinese team, does not match the chemical signature of mercury in the trench organisms, according to Blum and his colleagues.
They conclude that most of the mercury in the trench organisms was transported there in the carcasses of fish that feed in sunlit near-surface waters, where most of the mercury comes from anthropogenic sources.
“We studied the trench biota because they live in the deepest and most remote place on Earth, and we expected the mercury there to be almost exclusively of geologic origin—that is, from deep-sea volcanic sources,” Blum says. “Our most surprising finding was that we found mercury in organisms from deep-sea trenches that shows evidence for originating in the sunlit surface zone of the ocean.”
Anthropogenic mercury enters the oceans via rainfall, dry deposition of windblown dust, and runoff from rivers and estuaries.
“Deep-sea trenches have been viewed as pristine ecosystems unsullied by human activities. But recent studies have found traces of anthropogenic lead, carbon-14 from nuclear weapons testing, and persistent organic pollutants such as PCBs in organisms living in even the deepest part of the ocean, which is known as the hadal zone,” Drazen says.
The latest mercury findings provide yet another example of human activities affecting food webs in the most remote marine ecosystems on Earth.
Additional coauthors are from the University of Hawaii at Manoa, the University of Michigan, and Newcastle University.
Support for the research came from the Schmidt Ocean Institute, the National Science Foundation, and an NSF graduate fellowship.
Source: University of Michigan