New research may shed light on how we form and retain memories.
Scientists simulated the mechanics of a complex network that helps give neurons their ever-changing structures. They found the complex, Arp2/3, may be largely responsible for the “avalanches” observed in the cells’ cytoskeletal networks.
The research follows a study last year that detailed the interactions that allow neurons to accept the electrical signals that remodel their structures. An earlier study suggested actin filaments that control the shape of neurons may be the key to the formation and storage of long-term memories.
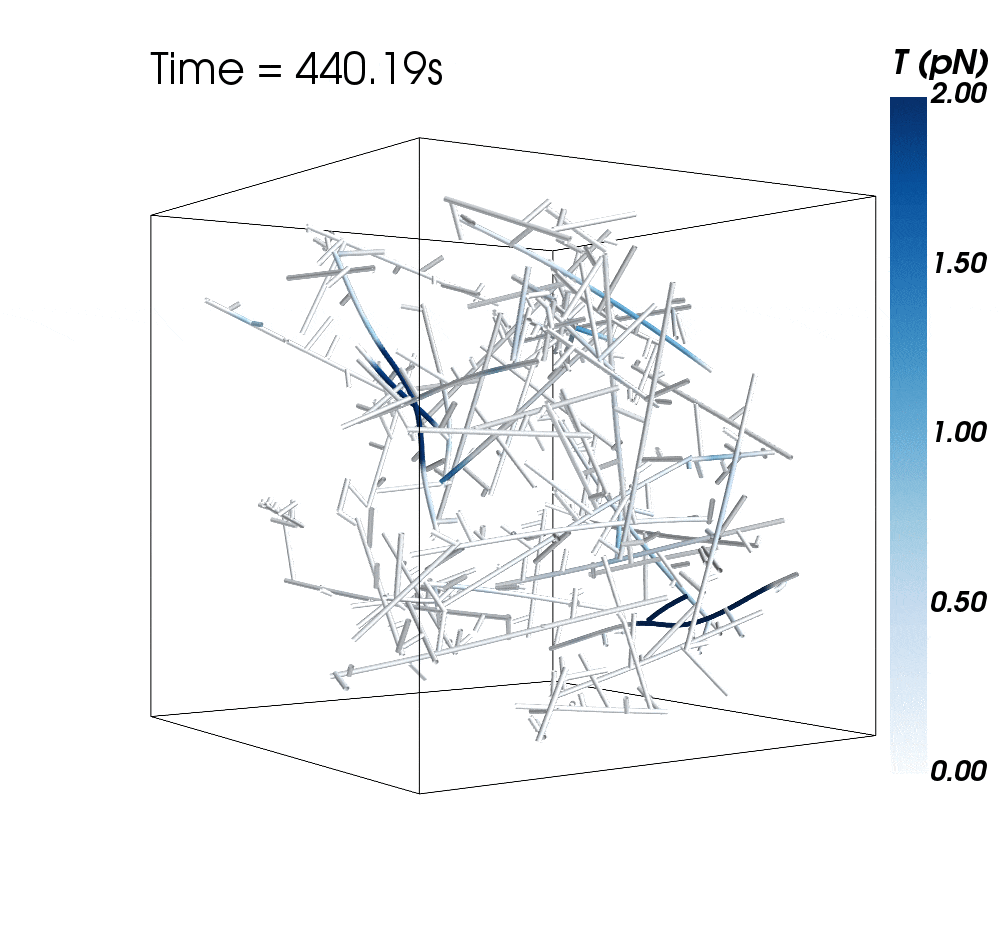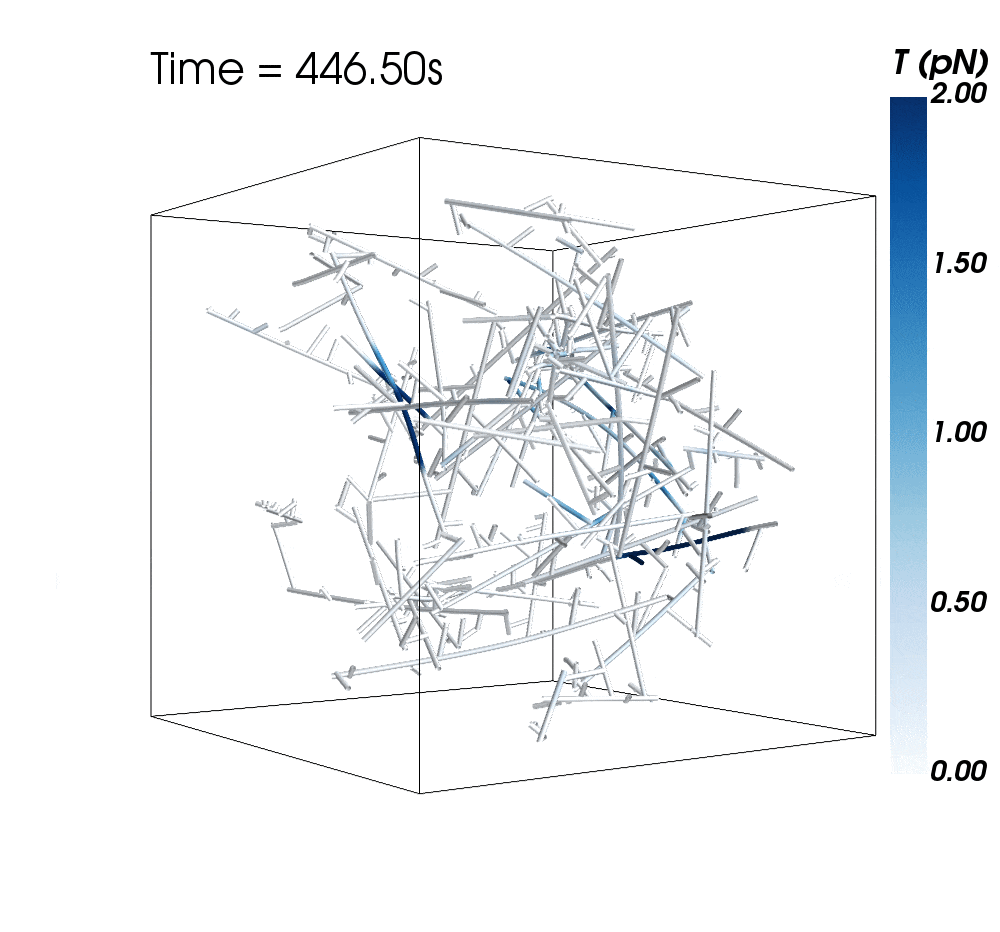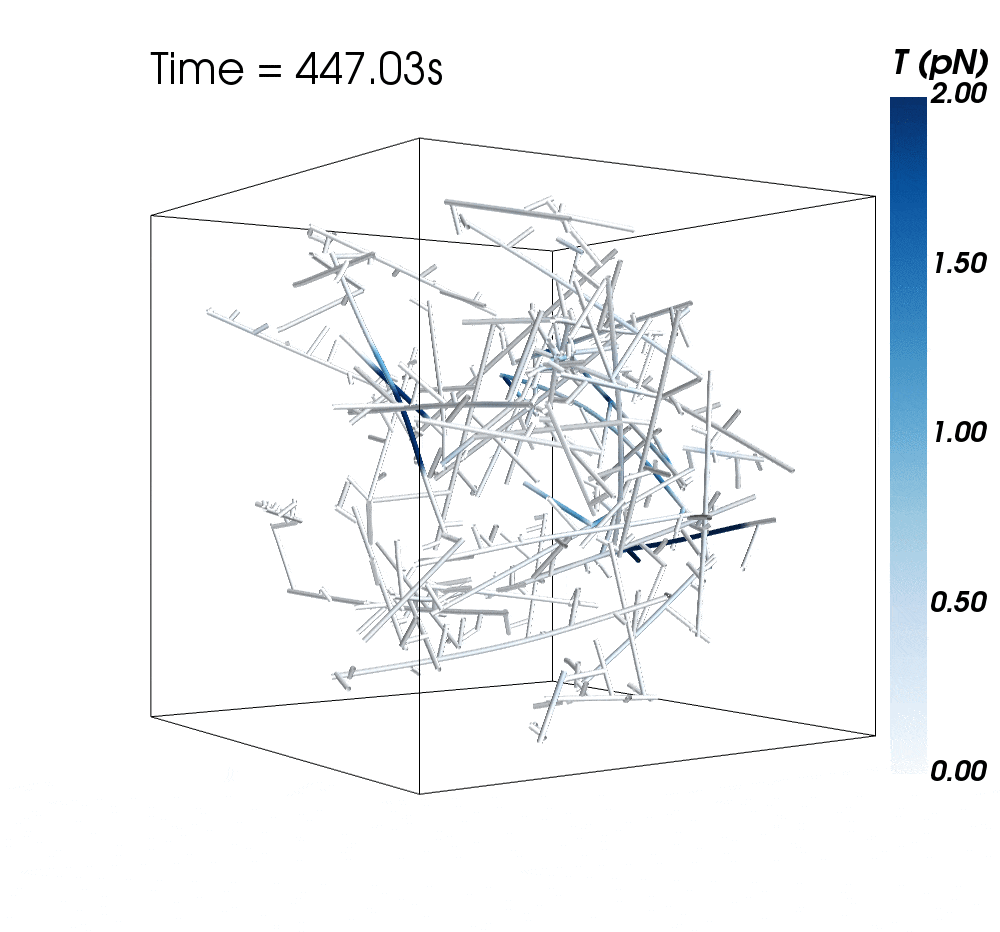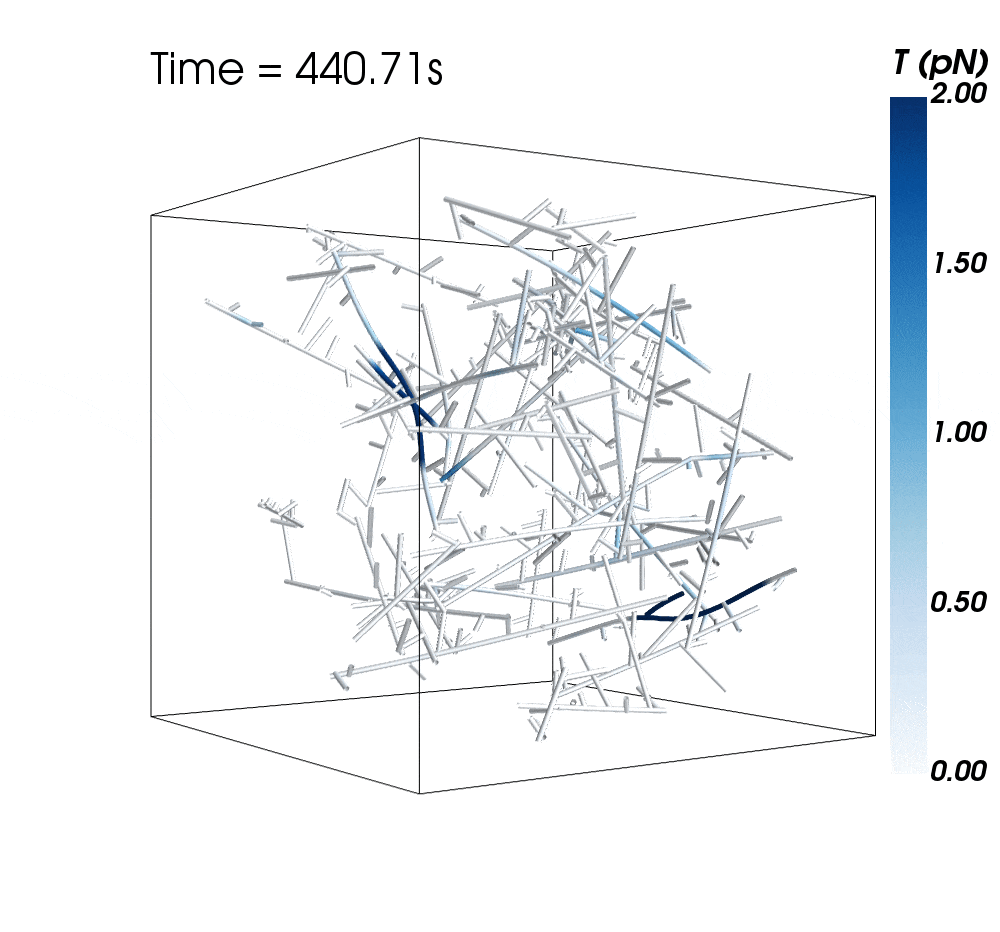
The new research suggests cytoskeletal avalanches within the neurons’ dendritic spines may be one way they retain new information.
Much about the cytoskeleton in every cell remains a mystery, but neurons are particularly interesting to the research team, which studies how they acquire information and store it for later use.
Lego-like actin proteins assemble to form these spidery filaments that allow motor proteins to carry nutrients and other cargoes across cells. They also give cells the ability to move and divide.
Sometimes, these filaments form branched actomyosin networks that researchers have observed collapsing. The simulations revealed the presence of Arp2/3 is key to nucleating branched actin networks that occasionally convulse and release strain in the network. (Arp stands for actin-related protein.)
When Arp2/3 was present, the simulations showed branched networks tended to relax significantly more slowly than unbranched networks do.
“There’s an analogy I use,” says Peter Wolynes, professor of chemistry, material science and nano-engineering, and physics and astronomy at Rice University. “With memory, you have to have something that changes, then it has to remain relatively permanent, but then perhaps be able to change again.
“Suppose you have a pillow made up of a random array of feathers,” he says. “They’re basically rods, similar to the branched structure of actin. If you put your head on the pillow, you crush it down, and when you get up later it still has that same crushed shape. Another time, it could have a different shape. So it has memory.”
Actin networks retain memory in somewhat the same way, Wolynes says. “Like your pillow, the rods in the network reconfigure when you put stress on them, in this case, an electrochemical signal input. When signaled, they undergo a series of avalanches that change the shape of the dendritic spine.”
“These are also similar to earthquakes in a sense,” Levine adds. “In an earthquake, the ground is static for a long time and then you have a dramatic event that reconfigures things. This new configuration lasts for a long time.
“The novelty of what’s being done here is that we’re not just focusing on individual molecules, as we’ve often done in the past,” he says. “We’re figuring out how individual molecules and their properties can modulate structures at larger length scales.”
The models showed branched actomyosin networks do not destabilize at specific concentrations, but that the avalanches depend on the initial configuration of the network as well as the history of past avalanches.
Margaret Cheung, a physicist at the University of Houston, whose lab continues to run three-dimensional MEDYAN models that combine mechanics and chemistry to study actomyosin dynamics, says many additional proteins are involved and are being studied by the team in order to understand memory.
The team hopes to tie its new findings to the earlier study led by Wolynes on how actin filaments exert force to stabilize long-term memories in prionlike fibers.
“One of the ways in which memory becomes more lasting is to change the global shape of the dendritic spine,” says biophysicist Herbert Levine. “That’s not currently in our modeling framework, and it will be an extensive effort to get it to work, but I’m interested in how to extend these models to calculate how the shapes change.”
The findings will appear in the Proceedings of the National Academy of Sciences.
Additional coauthors are from Rice, University of Houston, Northeastern University, and the University of Texas Health Science Center at Houston’s McGovern Medical School. The National Science Foundation supported the research.
Source: Rice University



