In bioelectronics breakthrough, scientists have created soft, flexible semiconductors.
The ideal material for interfacing electronics with living tissue is soft, stretchable, and just as water-loving as the tissue itself: in short, a hydrogel.
Meanwhile, semiconductors—the key materials for bioelectronics such as pacemakers, biosensors, and drug delivery devices—are traditionally known to be rigid, brittle, and water-hating.
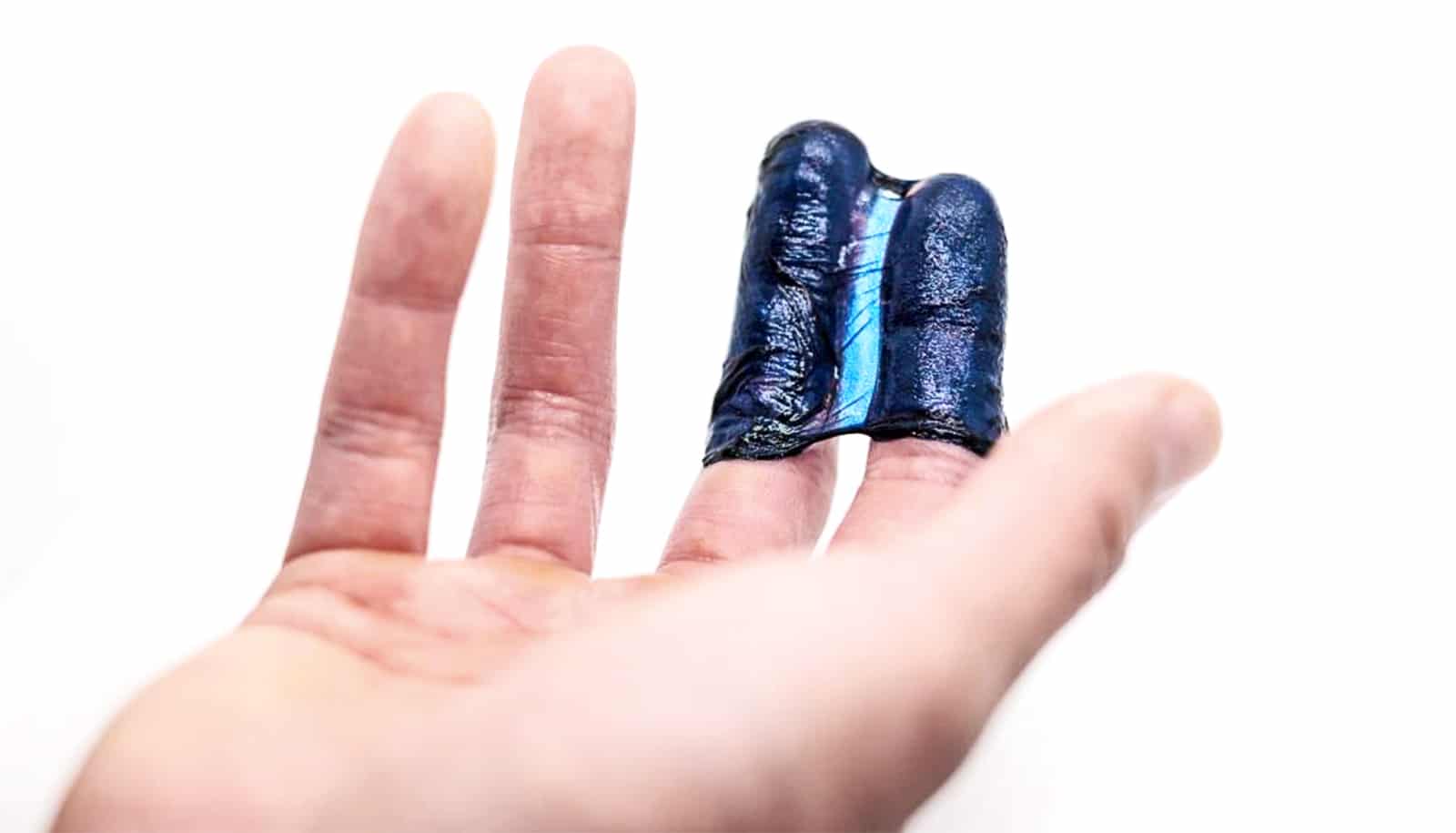
A paper published in Science has solved this challenge that has long stymied researchers. The result is a bluish gel that flutters like a sea jelly in water, but retains the immense semiconductive ability needed to transmit information between living tissue and machine.
This means their material—both semiconductor and hydrogel at the same time—ticks all the boxes for an ideal bioelectronic interface.
“When making implantable bioelectronic devices, one challenge you must address is to make a device with tissue-like mechanical properties,” says Yahao Dai, the first author of the new paper.
“That way, when it gets directly interfaced with the tissue, they can deform together and also form a very intimate bio-interface.”
Although the paper mainly focused on the challenges facing implanted medical devices such as biochemical sensors and pacemakers, Dai says the material also has many potential nonsurgical applications, like better readings off the skin or improved care for wounds.
“It has very soft mechanical properties and a large degree of hydration similar to living tissue,” says Sihong Wang, an assistant professor at the University of Chicago Pritzker School of Molecular Engineering, whose lab led the research. “Hydrogel is also very porous, so it allows the efficient diffusion transport of different kinds of nutrition and chemicals.
“All these traits combine to make hydrogel probably the most useful material for tissue engineering and drug delivery.”
The typical way of making a hydrogel is to take a material, dissolve it in water, and add gelation chemicals to puff the new liquid into a gel form. Some materials simply dissolve in water, others require researchers to tinker and chemically modify the process, but the core mechanism is the same: No water, no hydrogel.
Semiconductors, however, don’t normally dissolve in water. Rather than find new, time-consuming means of trying to force the process, the researchers re-examined the question.
“We started to think, ‘Okay, let’s change our perspective,’ and we came up with a solvent exchange process,” Dai says.
Instead of dissolving the semiconductors in water, they dissolved them in an organic solvent that is mixable with water. They then prepared a gel from the dissolved semiconductors and hydrogel precursors.
An important benefit of such a solvent-exchange-based method is its broad applicability to different types of polymer semiconductors with different functions.
The hydrogel semiconductor, which the team has patented and is commercializing through UChicago’s Polsky Center for Entrepreneurship and Innovation, is not merging a semiconductor with a hydrogel. It’s one material that is both semiconductor and hydrogel at the same time.
“It’s just one piece that has both semiconducting properties and hydrogel design, meaning that this whole piece is just like any other hydrogel,” Wang says.
Unlike any other hydrogel, however, the new material actually improved biological functions in two areas, creating better results than either hydrogel or semiconductor could accomplish on their own.
First, having a very soft material bond directly with tissue reduces the immune responses and inflammation typically triggered when a medical device is implanted.
Second, because hydrogels are so porous, the new material enables elevated biosensing response and stronger photo-modulation effects. With biomolecules being able to diffuse into the film to have interactions, the interaction sites for biomarkers-under-detection are significantly increased, which gives rise to higher sensitivity.
Besides sensing, the responses to light for therapeutic functions at tissue surfaces also get increased from the more efficient transport of molecules. This benefits functions such as light-operated pacemakers or wound dressing that can be more efficiently heated with a flick of light to help speed healing.
“It’s a ‘one plus one is greater than two’ kind of combination,” Wang jokes.
Funding for the work came from the US National Institutes of Health, the US Office of Naval Research, and the University of Chicago start-up fund.
Source: Paul Dalling for University of Chicago