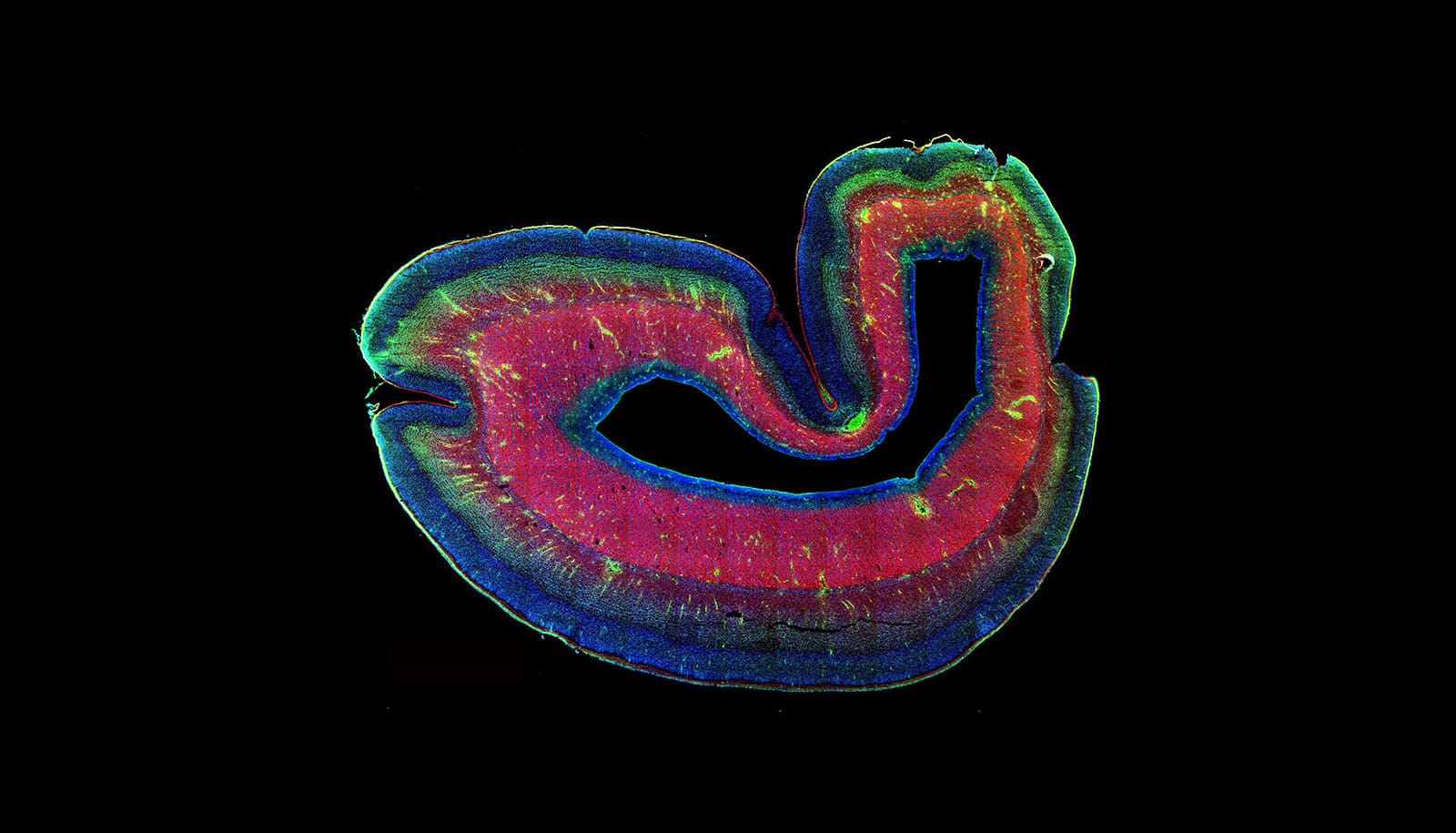
Support cells in the brain are key contributors to Huntington’s disease, according to a new study.
The research gives scientists a clearer picture of what is happening in the brains of people with the disease and lays out a potential path for treatment.
“Huntington’s is a complex disease that is characterized by the loss of multiple cell populations in the brain,” says Steve Goldman, codirector of the Center for Translational Neuromedicine at the University of Rochester Medical Center and lead author of the paper, which appears in Cell Stem Cell.
“These new findings help pinpoint how the genetic flaw in Huntington’s gives rise to glial cell dysfunction, which impairs the development and role of these cells, and ultimately the survival of neurons,” Goldman says.
“While it has long been known that neuronal loss is responsible for the progressive behavioral, cognitive, and motor deterioration of the disease, these findings suggest that it’s glial dysfunction which is actually driving much of this process.”
Failing support
Huntington’s is a hereditary and fatal neurodegenerative disease characterized by the loss of medium spiny neurons, a nerve cell in the brain that plays a critical role in motor control.
As the disease progresses over time and more of these cells die, the result is involuntary movements, problems with coordination, and cognitive decline, depression, and often psychosis. There is currently no way to slow or modify the progression of this disease.
While the symptoms of the disease are the result of the loss of motor neurons, the new study adds to mounting evidence that faulty glia, the brain’s primary support cells may trigger the disease.
These cells include oligodendrocytes—which produce the brain’s supply of myelin, an insulating substance that mediates communication between nerve cells—and astrocytes, which support the function of neurons and maintain the chemical balance necessary for nerve cells to communicate with their neighbors.
Slowing the disease
Earlier clinical studies have hinted at the role of glial cells in the disease. MRI scans of patients with Huntington’s have shown myelin and white matter loss in the brain long before the symptoms of the disease appear.
For the news study, researchers used embryonic stem cells from individuals with the Huntington’s disease mutation and reprogrammed them to become glial progenitors—the parent cell that gives rise to both astrocytes and oligodendrocytes.
When these cells are transplanted into the brains of mice, they out-compete native cells and produce brains with human glia and animal neurons. Researchers have also used the model to study other diseases in which it is believe that glial cells play a role, including frontotemporal dementia and schizophrenia.
In 2016, Goldman’s lab found that, when researchers transplanted healthy human glial cells into mice they engineered to express the mutant Huntington gene, the progress of the disease slowed. That research focused on astrocytes which, among other functions, serve as a buffer for potassium and help regulate the electrical signals that form at synapses, the points of connection between nerve cells.
Genetic code stutter
When astrocytes are not doing their job, as is the case in Huntington’s disease, the resulting chemical imbalance causes neurons to become overexcited and ultimately die. In those experiments, the new healthy human astrocytes restored normal neuronal activity and rescued nerve cells that might otherwise have died.
The new research gives scientists a more precise understanding of how the genetic flaws in people with the disease result in dysfunctional glial cells. A genetic mutation called a repeat expansion, or a stutter that results in duplicates of a small section of genetic code causes Huntington’s. The length of this repeat can dictate the age of onset and severity of the disease.
Using cells derived from individuals with the Huntington’s mutation, researchers were able to identify a series of errors that impeded the transcription of genetic information into proteins that tell progenitor cells to become either oligodendrocytes or astrocytes.
In Huntington’s disease these transcription errors impaired the creation of oligodendrocytes, and resulted in fewer cells able to make myelin, and also affected the production of astrocytes, which failed to mature properly and were unable to effectively regulate the chemical agents necessary for communication between nerve cells.
The research suggests that viral vectors or gene editing techniques like CRISPR could activate the transcription of these deficient glial genes. Goldman’s lab tested this approach in mutant Huntington mice, and found that forced expression of the genes restored myelination in the animals’ brains.
The study also validated earlier findings that placed glial cell dysfunction at the center of the disease and the idea that replacing sick glial cells with healthy ones may offer the most direct way to halt the disease’s progression.
Additional coauthors are from the University of Rochester and the University of Copenhagen. The National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the CHDI Foundation, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Lundbeck Foundation, and the Novo Nordisk Foundation funded the work.
Source: University of Rochester