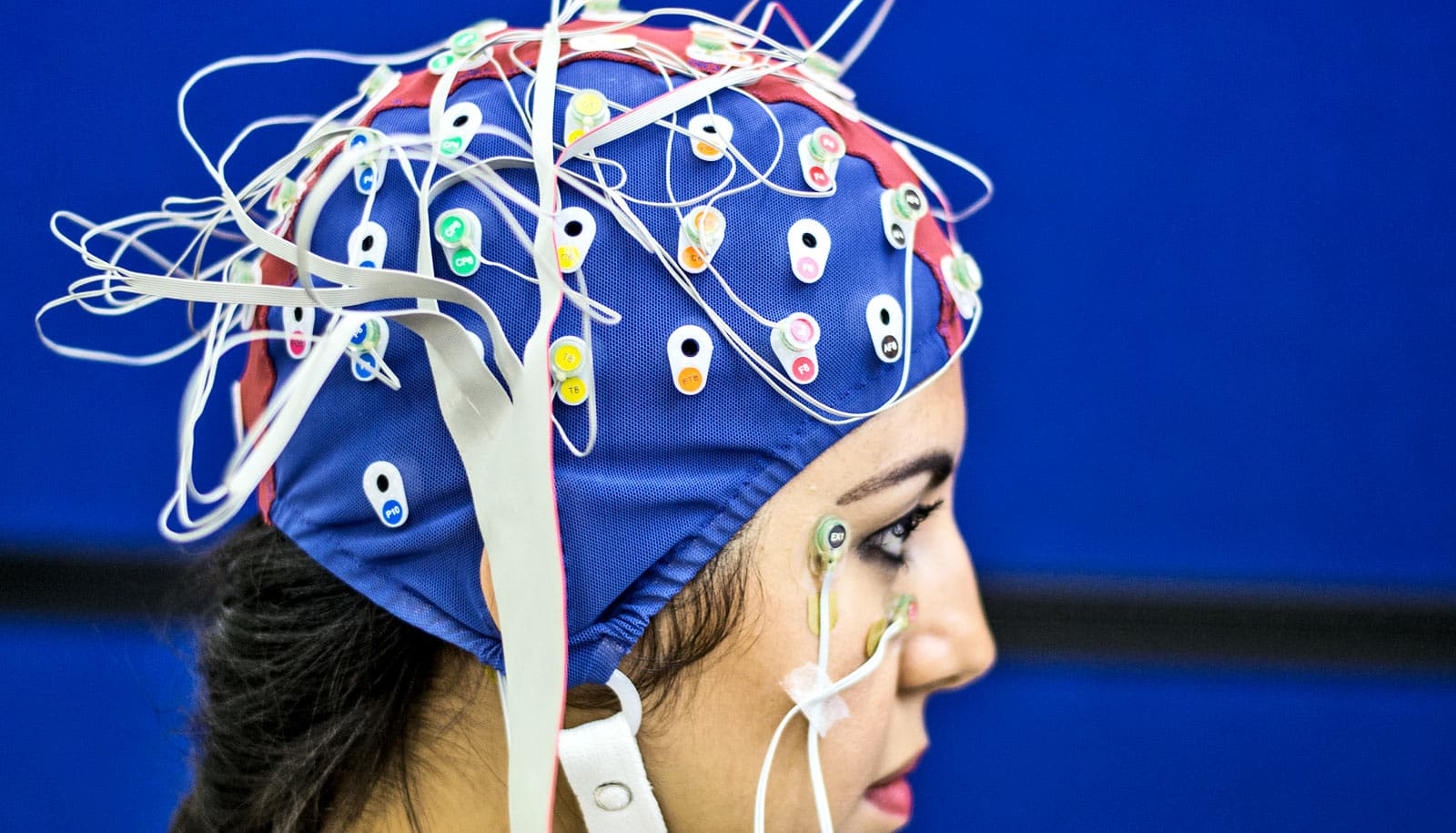
Two new models could solve a problem that’s long frustrated millions of people with epilepsy and the doctors who treat them: how to find precisely where seizures originate to treat exactly that part of the brain.
By helping surgeons decide if and where to operate, the tools could help patients avoid risky and often-ineffective surgeries as well as prolonged hospital stays.
“If you find that zone and you effectively treat it, it’s a game changer…”
“These are underserved patients,” says Sridevi V. Sarma, associate director of Johns Hopkins Institute of Computational Medicine and head of the Neuromedical Control Systems Lab. “We want surgeries to go well, but we also want to prevent surgeries that may never go well.”
Using equations based on machine learning and calculus to reveal patterns in brain activity, the models identify where seizures begin in the brain. And they do it in just minutes.
Typically patients spend five to 14 days hospitalized with electrodes stuck to their heads, while doctors hope that they’ll have a seizure so that surgeons can map the brain, pinpoint the trouble spot, and plan how to remove it.
“This is a new paradigm,” says Joon-Yi Kang, a neurologist at Johns Hopkins Hospital, who cowrote the studies. “We’re getting more insights into specific brain networks. We’re not waiting around for seizures to happen.”
More than 65 million people in the world have epilepsy, a condition makes them three times more likely to die. While most patients respond to medication, about 30% have drug-resistant epilepsy. Two treatment options are available to them: a device implanted into the brain to stop seizures with stimulation, or surgery to remove or disconnect brain regions where seizures originate.
Worse yet, surgery is only effective about half the time because it’s so difficult to identify where seizures start, Sarma says.
“If you find that zone and you effectively treat it, it’s a game changer—it’s a life-changing treatment for these patients,” she says.
To create heat maps predicting where seizures begin, Sarma’s team studied patients’ brains both when they weren’t having seizures and when their brains were stimulated with quick electrical pulses.
In their models, the brain is a network of nodes that influence each other. The researchers hypothesize that when a patient isn’t having a seizure, it’s because the nodes in the part of the brain where seizures begin are constrained by nodes in the healthy part of the brain. During a seizure, the nodes switch roles.
By identifying the strength and direction of the nodes, the researchers pinpointed where seizures began, says coauthor Kristin Gunnarsdottir, a Johns Hopkins research scientist.
In 65 patients studied, the model predicted the onset of seizures and the ultimate success of a surgery with 79% accuracy. “If we compare that to the traditional 50% success rate of surgeries, this could really help clinicians,” says Gunnarsdottir.
In the companion study of 28 patients, the researchers figured out which nodes were influencing the others by zapping the brain with a stimulation pulse.
“We’re hoping that this can be something that we could use in patients that don’t have a ton of seizures or in the 10% of patients that don’t have seizures at all during (traditional) monitoring,” says coauthor Rachel June Smith, a former postdoctoral fellow in biomedical engineering at Johns Hopkins who is now an assistant professor at the University of Alabama. Additional clinical trials are planned.
The study is published in the journal Brain. Additional coauthors are from the University of Pittsburgh Medical Center and Johns Hopkins University and Medicine.
The American Epilepsy Society, the National Institutes of Health, the National Institute of Neurological Disorders and Stroke and a NIH IRACDA Fellowship through the ASPIRE program at Johns Hopkins funded the work.
Source: Johns Hopkins University