A genetic variation in a gene called TMEM106B could explain why similar levels of head trauma can cause some people to suffer more drastic symptoms of chronic traumatic encephalopathy (CTE) than others, according to a new study.
After 10 years of studying brains that families of deceased military service people, football players, and other contact-sport athletes donated, researchers have amassed more than 600 brains, a collection that has grown large enough to allow meaningful analysis of the genetics related to CTE.
“In order to understand how genetic variants influence disease or severity, you need hundreds of cases,” says Ann McKee, professor of neurology and pathology at the Boston University School of Medicine, chief of neuropathology at the VA Boston Healthcare System, and director of the BU CTE Center.
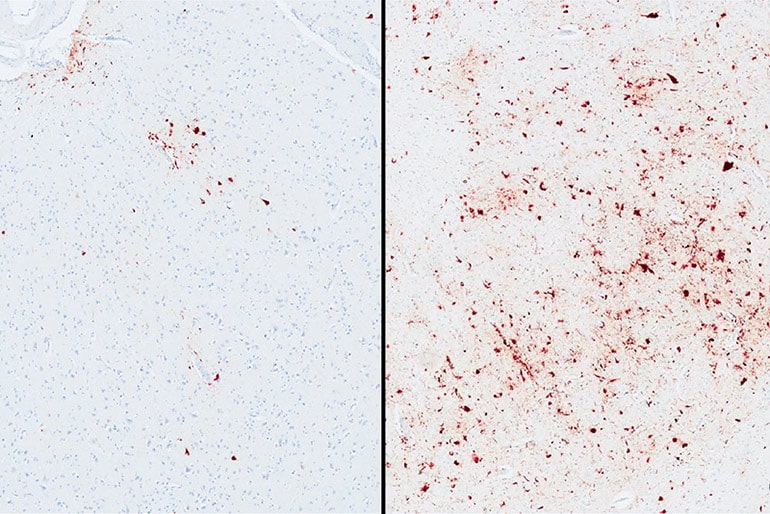
With more than 600 participants in the brain bank and counting, “we’ve reached a critical mass,” says McKee, co-senior author of the paper, which appears in Acta Neuropathologica Communications. “I expect to find a flood of new genes that influence the pathology of CTE. This is just the beginning.”
No needle-in-the-haystack discovery
Currently, studying a person’s brain after death is the only way to diagnose CTE. And until now, it’s been unknown why repeated head injuries can lead to a range of mild to severe CTE symptoms between individual college and professional athletes.
The newly-discovered genetic association between CTE and TMEM106B could someday help predict which people, while they are still alive, are most likely to develop severe symptoms of CTE, including dementia and the buildup of phosphorylated tau (p-tau) protein, a feature also associated with other neurodegenerative disorders like ALS, Alzheimer’s disease, and Parkinson’s.
“Among athletes who developed CTE pathology, those with the ‘risk’ variation of TMEM106B were 2.5 times more likely to have dementia and increased levels of p-tau,” says co-first author Jesse Mez, assistant professor of neurology, and a member of both the Alzheimer’s Disease and CTE Centers at BU.
TMEM106B was hardly a needle-in-the-haystack discovery. The team had good reason to question a link between TMEM106B and CTE.
Repeat offenders
“We focused on this gene because of its role in altering brain pathology in other neurological diseases and its role in inflammatory responses,” says co-senior author Thor Stein, a physician and neuropathologist at VA Boston Healthcare System, MED assistant professor of pathology and laboratory medicine, as well as associate director of the neuropathology core of the BU Alzheimer’s Disease Center.
From the brain bank—which first started recruiting brain donations from families of deceased athletes in 2008 in coordination with the Concussion Legacy Foundation and its founder, former professional wrestler Chris Nowinski—the team analyzed TMEM106B and evidence of CTE in white football players, a sample size of 86 brain bank participants, all of whom had been diagnosed with CTE.
In the search for genetic factors associated with CTE, football players make ideal study subjects because their head injuries are frequent and relatively comparable across the population, in contrast to people who sustained more random head injuries from military-related blasts or other various traumas.
“Football players go through what we call ‘repetitive head impacts,'” Stein says.
Given TMEM106B’s association with other neurodegenerative diseases, the team also excluded brain bank participants who had evidence of other related diseases.
Strong connection
Looking at the 86 participants who met the eligibility criteria for their analysis, researchers performed genetic sequencing and identified two distinct categories of TMEM106B genetic variation within the group. They found a “major” genetic variation in about 60 percent of people and a “minor” genetic variation in the remaining 40 percent.
Then, they assessed the participants based on several different traits known to be associated with CTE symptoms, including the loss of synapses, dementia before death, and p-tau accumulation throughout the brain.
When they compared the results with data from people who did not have CTE, the team found that TMEM106B does not influence whether or not a person develops CTE.
But among people diagnosed with CTE, they found that the 60 percent with the major genetic variation were significantly more likely to have higher accumulation of p-tau, more severe brain inflammation associated with CTE symptoms, and dementia during their lifetimes.
Taken with previous studies linking TMEM106B to dementia, ALS, and Alzheimer’s and Parkinson’s diseases, the findings further strengthen a connection between the TMEM106B gene and neurodegeneration.
Clean the brain
Although it’s not precisely known how TMEM106B influences p-tau accumulation and inflammatory responses, researchers have a working hypothesis based on what is known.
The TMEM106B gene directs cells to produce proteins of the same name, TMEM106B. In turn, those are thought to play a role in the formation of cell organelles that are responsible for digesting enzymes.
Yet too much TMEM106B, which could occur as a result of genetic variation, can make enzyme digestion less efficient. Inside brain cells that play an important role in “cleaning” the brain of damage-related proteins like p-tau, abnormal enzyme digestion could result in the hallmark buildup of the damaging proteins seen in cases of CTE.
“We’re at a place where we clearly know that there’s a link between contact sports and CTE, but we don’t yet have a good sense of the magnitude of that relationship,” Mez says.
There’s likely a number of genes that contribute on various levels to bring someone from the neurotypical end of the spectrum to a diagnosis of severe CTE. With the number of brain bank participants continuing to creep above 600, more genetic factors related to CTE could be on the near horizon.
McKee, who was recently named one of Time magazine’s 100 Most Influential People of 2018, says the research around brains with CTE could one day influence the popularity of football in America, unless the rules of the game begin to change.
“As your sample size gets bigger, you’re able to identify genes that have smaller and smaller effects,” says co-first author Jonathan Cherry, a postdoctoral fellow at the CTE Center. “It’s an advantage for us that we’re not just operating on clinical diagnoses of dementia, for example, but that we have the actual CTE pathology in front of us to quantify what we’re seeing with the presence of certain genetic variations.”
Source: Kat J. McAlpine for Boston University



