Researchers are pivoting their work to tackle the many engineering problems associated with the global coronavirus pandemic.
“I’m glad I’m an engineer right now,” says Joyce Wong, professor of biomedical and materials science engineering at Boston University’s School of Engineering. “There are so many problems that need to be solved in this crisis and I can actually use my expertise to help.”
Wong, like many other engineers and researchers, is diving in to do what she can to mitigate the COVID-19 pandemic. These efforts are in addition to the first wave of help that gathered personal protective equipment (PPE) from labs—shuttered by Massachusetts Governor Charlie Baker’s stay-at-home advisory—to donate to healthcare workers.
Here are four ways that engineers are using technology to tackle the coronavirus pandemic:
1. A ‘box’ and a bracket
Wong started working on two projects after talking to her cousin, Steven Horng, an emergency medicine physician at Beth Israel Deaconess Medical Center (BIDMC).
“I started hearing about the PPE shortages from Steven, and then he started to tell me about more of the challenges healthcare workers are facing,” she says. “We’re getting close to the predicted peak of cases in Massachusetts, so I want to help out any way I can.”
Wong is now collaborating with engineers Enrique Gutierrez Wing and J. Gregory McDaniel on medical equipment designed to help contain the SARS-CoV-2 virus responsible for causing COVID-19 infections.
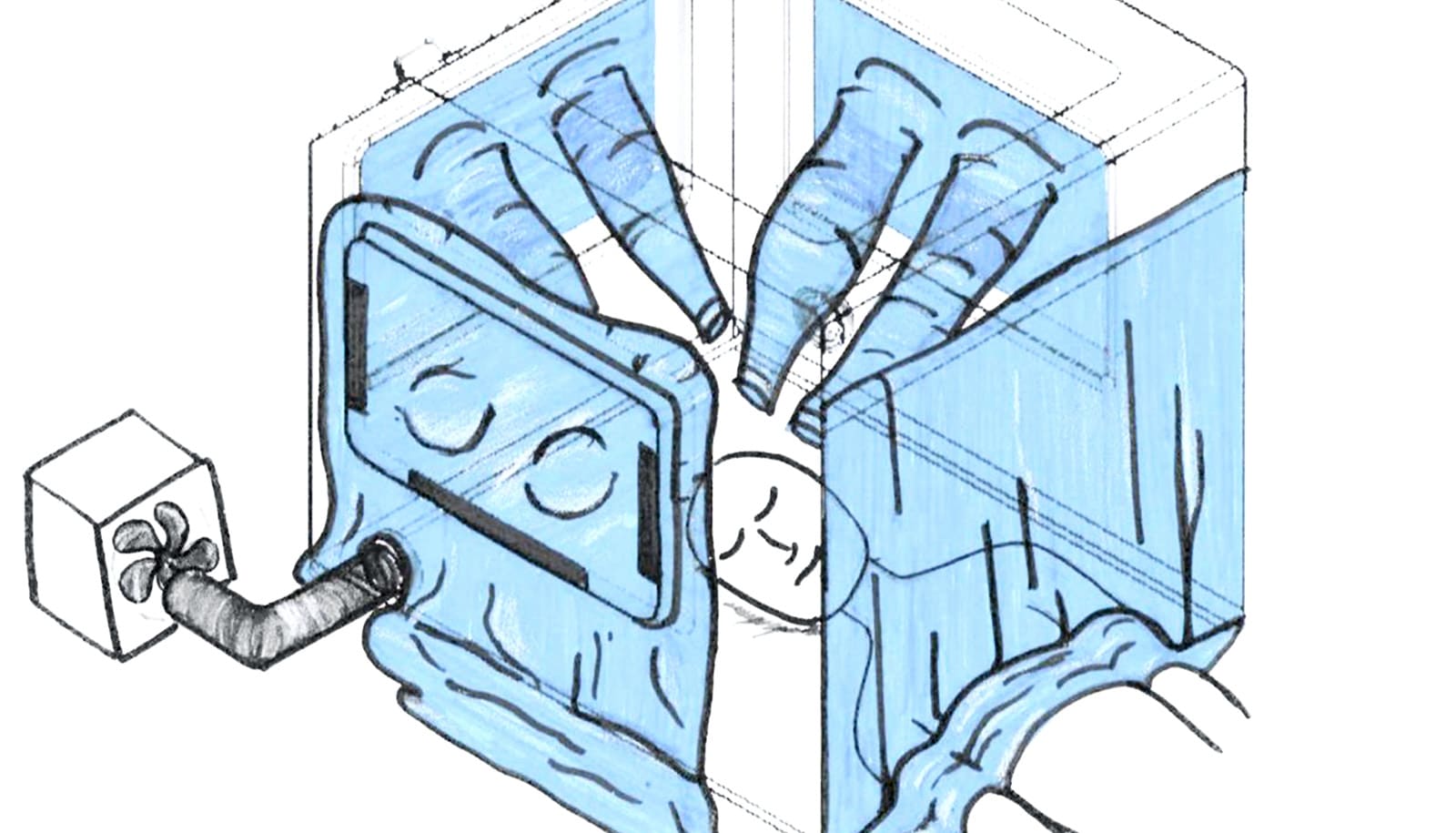
The first device is inspired by a photo Horng saw of an intubation box built by a Taiwanese doctor. Together, Horng and Wong teamed up with Gutierrez Wing and McDaniel to expand on that idea and make a negative pressure chamber, meant to safely isolate patients sickened by the virus and prevent their respiratory droplets from dissipating in the air outside the chamber. They also enlisted the help of Emily Whiting, a computer scientist and art technologist, and Patricia Fabian, an environmental health expert and infectious disease systems scientist.
The team has designed their so-called “respiratory isolation box” with features to allow doctors to maintain the same standard of care, but with an added layer of protection.
In addition to being a negative pressure chamber—meaning air doesn’t escape the box, but can flow in—it’s also designed so that up to three clinicians can work on one patient at a time. And the team is testing the box’s internal dimensions to make sure healthcare workers can still use the specialized equipment they may need when intubating a COVID-19 patient. The box is in the prototyping phase, but the team is collaborating with clinicians and planning to test the prototype in a medical simulation lab.
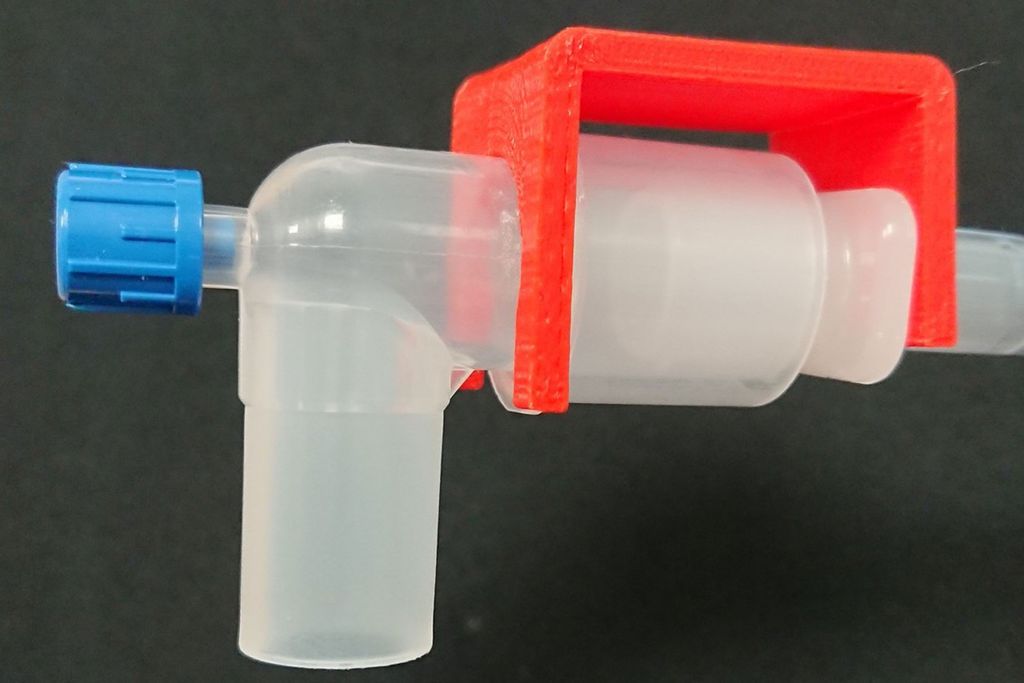
The second medical device that Wong, Gutierrez-Wing, and McDaniel are developing is a 3D-printed bracket designed to hold together an endotracheal tube and the respirator circuit it’s attached to.
In normal use, these connections between the ventilator machine and the tube that’s inserted down the patient’s throat are loose, intentionally made so that they can easily be disconnected in case the patient needs to be suddenly moved in an emergency situation. Sometimes, however, the loose connection comes apart randomly, disrupting ventilating and triggering an alarm that sends clinicians scrambling to reconnect it.
But in the case of patients receiving respiratory support due to COVID-19, when that disconnection happens, the air coming out of the tube and into the room is full of droplets containing the SARS-CoV-2 virus, leaving others at unnecessarily high risk of exposure and infection.
In contrast, the bracket that Wong’s team is developing easily clips into place to hold the tube and respirator hookup together to prevent this from happening. The 3D-printed bracket is made of a material that can be easily sterilized, and the prototype is designed with rounded edges so that it won’t tear clinicians’ latex gloves (which are now in especially short supply).
2. A new, faster COVID-19 test
Researchers are working to develop a rapid and reliable test for the SARS-CoV-2 virus.
The currently available tests look for the presence of SARS-CoV-2’s viral RNA, a unique and identifying genetic code. Building on previous research, the new test is fundamentally different: it detects and counts individual SARS-CoV-2 viruses by capturing them with antibodies.
The primary benefit of this approach is that its testing mechanism doesn’t require extensive sample preparation.
It also reduces the chance of false negative results. Viruses can mutate, but the currently available tests rely on knowing specific genetic sequences of the virus to detect it. So, if the coronavirus mutates within one of those sequences, a current test could report a false negative—which happened during the 2014 Ebola outbreak, making it difficult to accurately diagnose who was sick and contain the outbreak.
The new test uses a different set of supplies than the existing test, leaving it less prone to supply chain shortages than the current method, says Selim Ünlü, a professor of electrical, computer, materials science, and biomedical engineering, who is teaming up with John Connor, associate professor of microbiology at the School of Medicine, from the National Emerging Infectious Diseases Laboratories, and Mehmet Toner of Massachusetts General Hospital to develop the test.
3. Faster test validation
Catherine Klapperich, director of the Precision Diagnostics Center and a professor of biomedical and materials science engineering, is spearheading a team to validate new types of SARS-CoV-2 tests. To contain the current COVID-19 pandemic, and prevent future relapses, an extreme ramp up of testing is needed across the United States. But there are currently roadblocks and shortages of supplies barring that from being possible.
To increase testing capabilities, new tests must be evaluated and validated through FDA regulatory procedures. Those validations take time—so, Klapperich’s team is trying to speed up that process.
The Precision Diagnostics Center is taking on the task of preclinical lab validation of newly developed COVID-19 tests. First up, they’re working with one developed by Michael Springer’s systems biology group at Harvard Medical School.
Klapperich’s team has already validated a version of his test by seeing how well the method can be used to positively detect flu from a bank of H1N1 patient samples from the 2009-2010 pandemic.
Now with that proof of concept in hand, Springer is adapting the test to make it work for the SARS-CoV-2 virus. In the meantime, Klapperich is securing COVID-19 patient samples from collaborators at Boston Children’s Hospital to validate the next iteration of Springer’s assay as well as COVID-19 tests developed by other research groups.
4. Better nasal swabs
Because of the COVID-19 pandemic, the nationwide supply of nasal swabs used to collect patient samples are also at risk of running out. Engineers Mark Grinstaff, Sheila Russo, and Anna Thornton have teamed up to identify new viable materials that would enable highly effective nasal swabs to be fabricated via 3D printing.
Not only would their approach, using new materials, skirt the supply chain issue, it would also reduce the likelihood of false negative COVID-19 test results. Nasal swabs used for coronavirus tests must be pushed up a patient’s nose to collect a mucus sample from where the throat meets the back of the nose, which requires training and can be prone to user error. A swab made of a material that more easily collects mucus, increasing the chance of capturing a high-quality sample, would help reduce false negatives, the researchers say.
Source: Boston University