Developing cells retain a memory of the chemical signals they encounter, according to a new research. Embryos that fail to form these memories remain a clump of clones, never realizing their unique biological potential.
We all start out as a clump of identical cells. As these cells divide and multiply, they gradually take on distinct identities, acquiring the traits necessary to form, for instance, muscle tissue, bone, or nerves. The new study offers insight into how our cells cultivate these identities over the course of development.
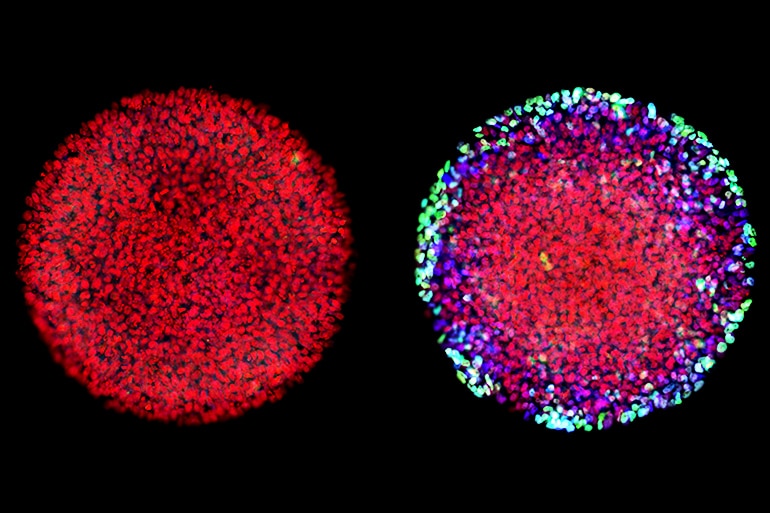
Cellular fate
Over 25 years ago, Ali H. Brivanlou demonstrated that the protein Activin causes embryonic frog cells to take on traits specific to certain tissue types, a process called differentiation. For decades now, Activin has been thought to instigate the transition from homogenous clump to specialized cells.
“Activin was the textbook definition of a molecule that is necessary and sufficient for differentiation,” says Brivanlou, a professor in the Laboratory of Stem Cell Biology and Molecular Embryology at Rockefeller University. “Researchers had shown that the dose of the protein determines cellular fate. At a very high dose, for example, you get gut and muscle; and at a very low dose, you get nerve tissue.”
Despite ample evidence from animal studies, questions remained about how Activin guides development in human cells. Researchers set out investigate whether the protein triggers differentiation in laboratory-generated human embryos. Developed from stem cells, these embryos mimic the behavior of human cells during the early stages of development.
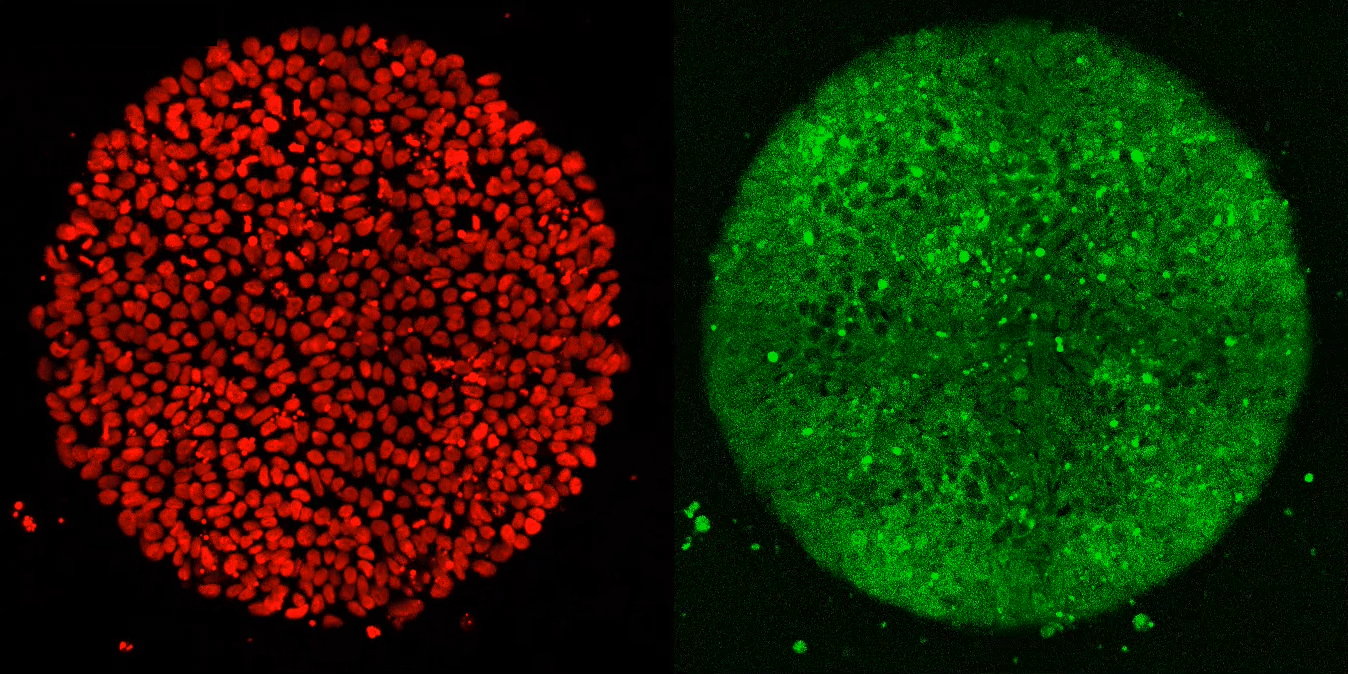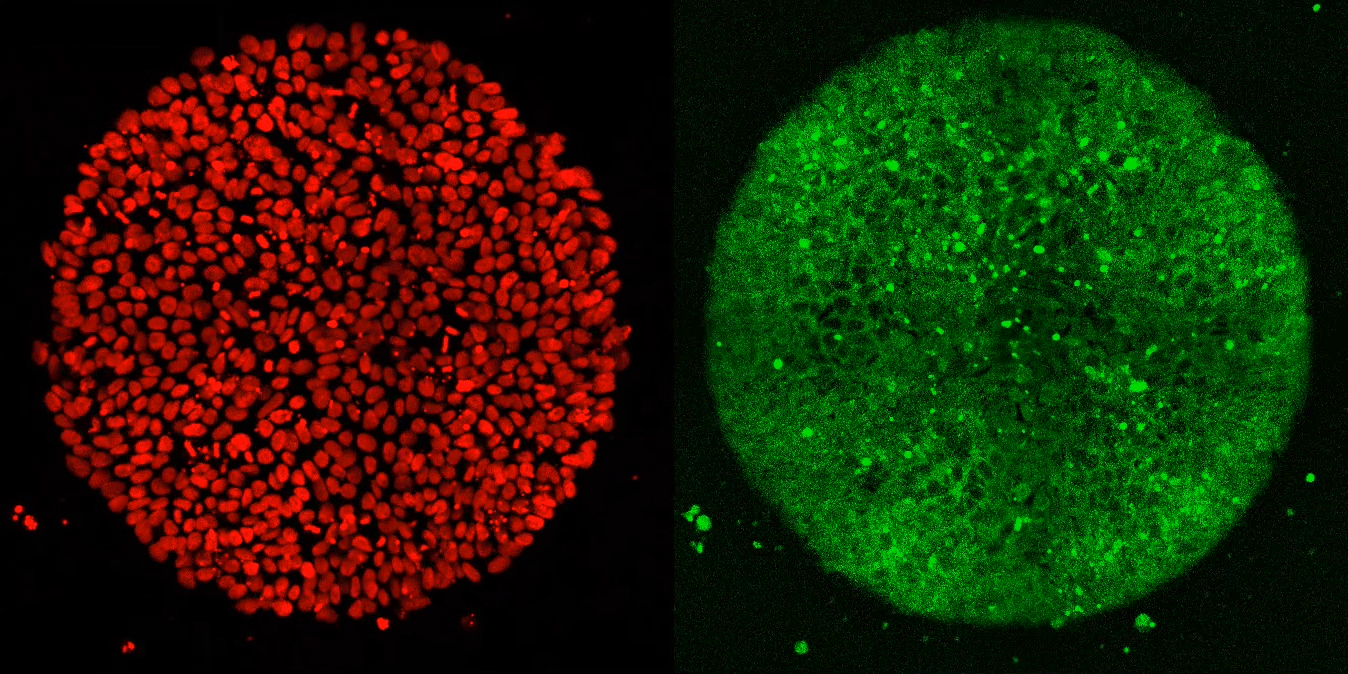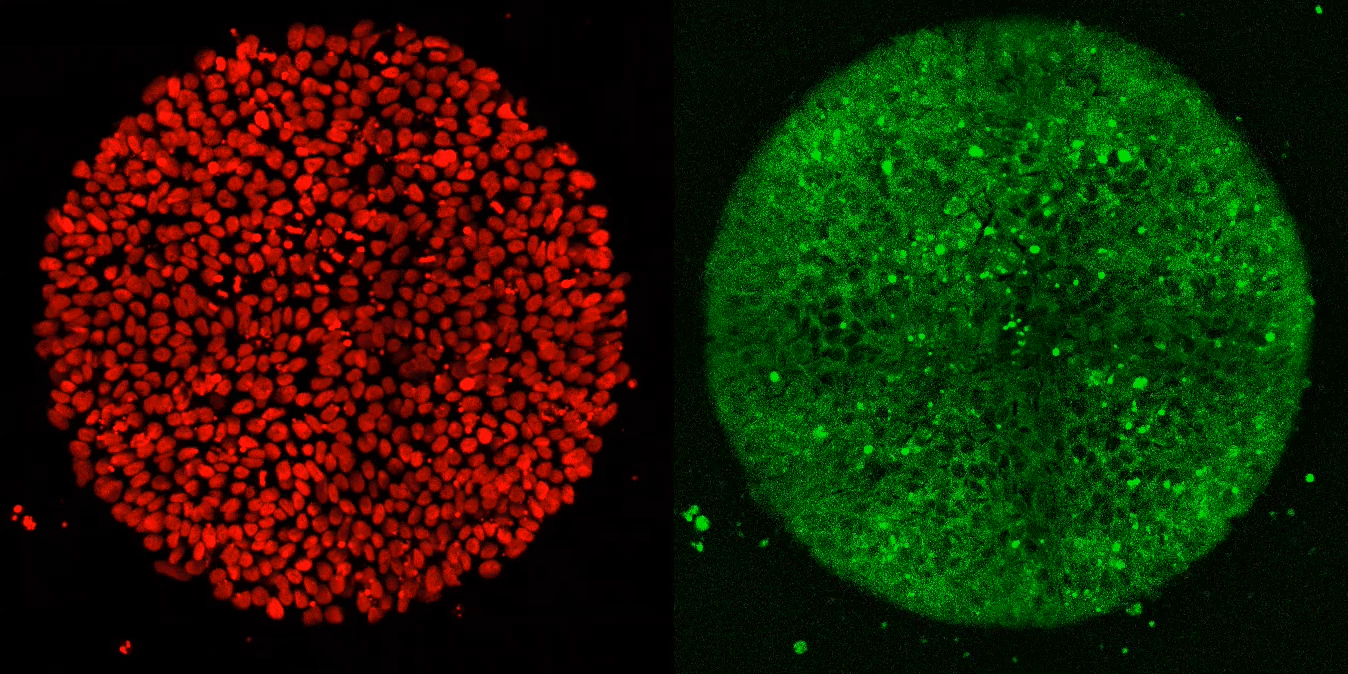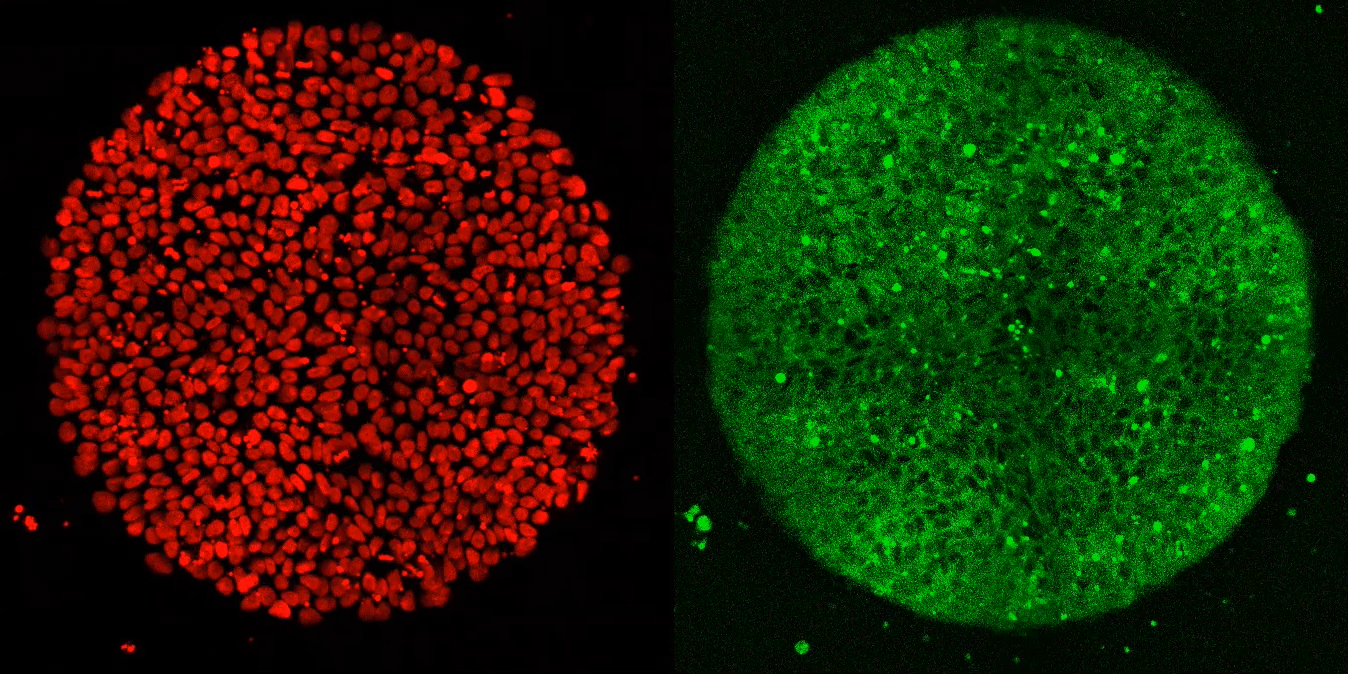
The researchers expected these synthetic embryos to respond just like Brivanlou’s frogs. Yet, after applying Activin to these cells, they observed, well, nothing.
“Anna (graduate fellow Anna Yoney) put Activin on the embryos and we waited—and waited and waited. And absolutely nothing happened! That was shocking,” says Brivanlou.
Making sense of the surprise
Undeterred, Yoney considered possible explanations for her results. “I thought, OK, we don’t get a response from Activin alone,” she recalls. “What additional signals might we need to see differentiation?”
She ultimately homed in on WNT, a molecule known to regulate the movement of cells during development. In her next experiment, she exposed the cells to WNT before adding Activin; and, this time, they differentiated in the normal manner.
“The cells that saw WNT reacted to Activin with the full range of response—just like we see in the frog and other animals,” says Brivanlou. “But cells that hadn’t seen WNT were totally unresponsive, as if Activin wasn’t even there.”
The researchers concluded that differentiation requires both WNT and Activin signaling. Crucially, however, they showed that cells don’t need exposure to the two chemicals simultaneously.
“We blocked WNT signaling during the Activin treatment phase and found that the cells still differentiated,” says Yoney. “So we concluded that the cells actually remembered that they had previously been exposed to WNT.”
‘Signaling memory’
The researchers deemed this phenomenon “signaling memory” because WNT appears to permanently change cells that cross its path. Earlier research in this area failed to uncover evidence for embryonic memories because, says Brivanlou, most developmental biologists work with animal cells.
“In animal model systems, cells encounter a series of signals before people like me manipulate them. But Anna’s artificial embryos came from stem cells that hadn’t had this kind of exposure—and this makes them perfect tools for discovering the roles of other signals,” he says. “As beautiful as the model systems are, sometimes they can lead you to miss things.”
The researchers hope to further explore how and where cells store memories. Yoney suspects that they are recorded in cells’ nuclei as modifications to the epigenome, which controls the way that cells read out their DNA. Additional research in this area could have major implications for understanding development in humans and other species.
The study appears in eLife.
Source: Rockefeller University


