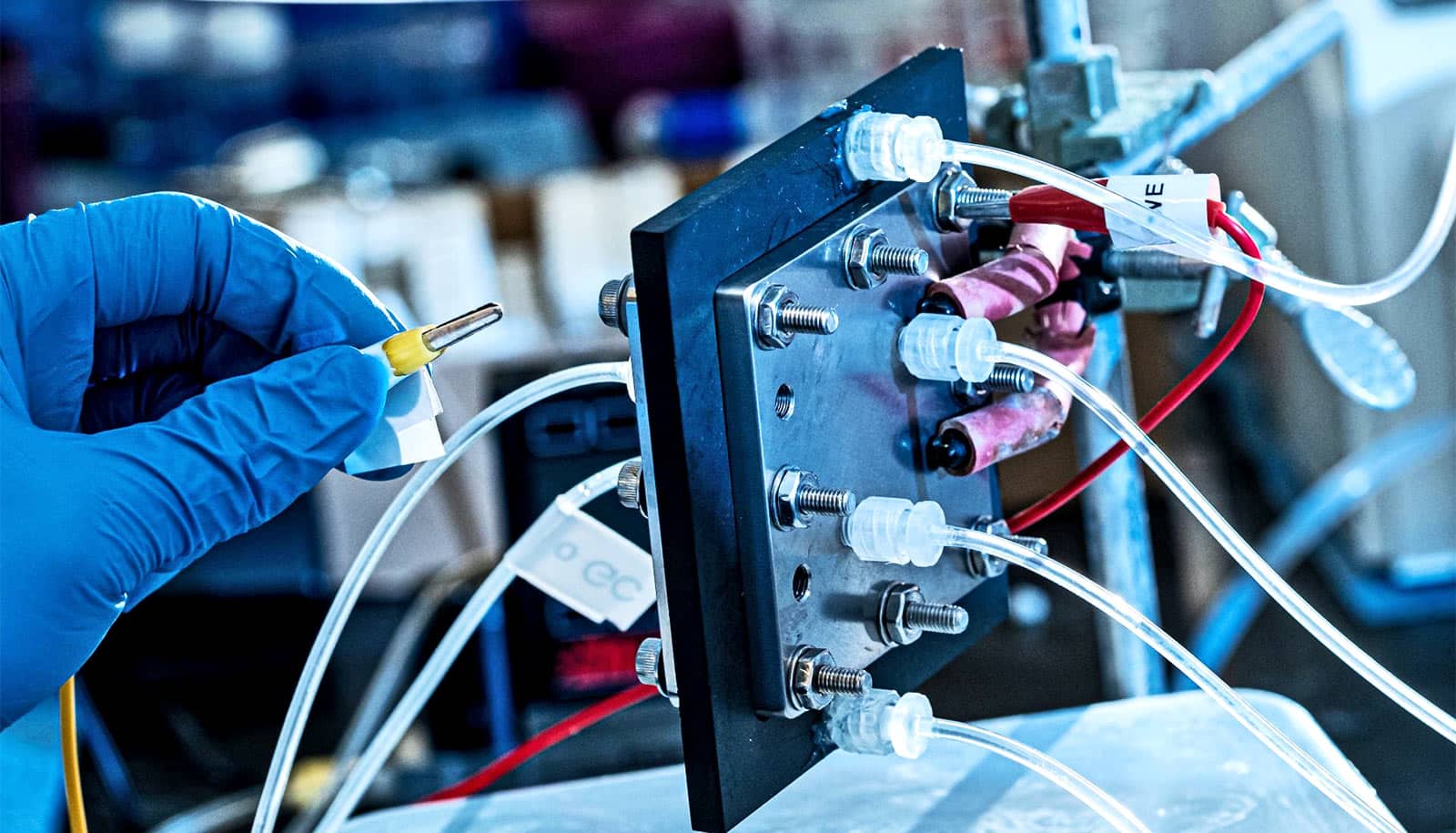
An electrolyzer that uses renewable electricity could produce pure liquid fuels from carbon dioxide in an efficient and environmentally friendly way, researchers report.
In its latest prototype, the catalytic reactor produces highly purified and high concentrations of formic acid.
Formic acid that traditional carbon dioxide devices produce needs costly and energy-intensive purification steps, says Haotian Wang, a chemical and biomolecular engineer at Rice University. The direct production of pure formic acid solutions will help promote commercial carbon dioxide conversion technologies.
Taking carbon dioxide from waste to ‘wow’
Wang’s lab developed the reactor, and his group pursues technologies that turn greenhouse gases into useful products. In tests, the new electrocatalyst reached an energy conversion efficiency of about 42%. That means nearly half of the electrical energy can be stored in formic acid as liquid fuel.
“Formic acid is an energy carrier,” Wang says. “It’s a fuel-cell fuel that can generate electricity and emit carbon dioxide—which you can grab and recycle again.
“It’s also fundamental in the chemical engineering industry as a feedstock for other chemicals, and a storage material for hydrogen that can hold nearly 1,000 times the energy of the same volume of hydrogen gas, which is difficult to compress,” he says. “That’s currently a big challenge for hydrogen fuel-cell cars.”
Key advancements
Two advances made the new device possible, says lead author Chuan Xia, a postdoctoral fellow. The first was his development of a robust, two-dimensional bismuth catalyst and the second a solid-state electrolyte that eliminates the need for salt as part of the reaction.
“Bismuth is a very heavy atom, compared to transition metals like copper, iron, or cobalt,” Wang says. “Its mobility is much lower, particularly under reaction conditions. So that stabilizes the catalyst.” He notes the reactor is structured to keep water from contacting the catalyst, which also helps preserve it.
Xia can make the nanomaterials in bulk. “Currently, people produce catalysts on the milligram or gram scales,” he says. “We developed a way to produce them at the kilogram [2.2 lbs] scale. That will make our process easier to scale up for industry.”
The polymer-based solid electrolyte is coated with sulfonic acid ligands to conduct positive charge or amino functional groups to conduct negative ions.
“Usually people reduce carbon dioxide in a traditional liquid electrolyte like salty water,” Wang says. “You want the electricity to be conducted, but pure water electrolyte is too resistant. You need to add salts like sodium chloride or potassium bicarbonate so that ions can move freely in water.
“But when you generate formic acid that way, it mixes with the salts,” he says. “For a majority of applications you have to remove the salts from the end product, which takes a lot of energy and cost. So we employed solid electrolytes that conduct protons and can be made of insoluble polymers or inorganic compounds, eliminating the need for salts.”
The rate at which water flows through the product chamber determines the concentration of the solution. Slow throughput with the current setup produces a solution that is nearly 30% formic acid by weight, while faster flows allow researchers to customize the concentration. The researchers expect to achieve higher concentrations from next-generation reactors that accept gas flow to bring out pure formic acid vapors.
“X-ray absorption spectroscopy, a powerful technique available at the Inner Shell Spectroscopy (ISS) beamline at Brookhaven Lab’s National Synchrotron Light Source II, enables us to probe the electronic structure of electrocatalysts in operando—that is, during the actual chemical process,” says coauthor Eli Stavitski, lead beamline scientist at ISS. “In this work, we followed bismuth’s oxidation states at different potentials and were able to identify the catalyst’s active state during carbon dioxide reduction.”
100 hours of power
With its current reactor, the lab generated formic acid continuously for 100 hours with negligible degradation of the reactor’s components, including the nanoscale catalysts.
Wang suggests researchers could easily retool the reactor to produce such higher-value products as acetic acid, ethanol, or propanol fuels.
“The big picture is that carbon dioxide reduction is very important for its effect on global warming as well as for green chemical synthesis,” Wang says. “If the electricity comes from renewable sources like the sun or wind, we can create a loop that turns carbon dioxide into something important without emitting more of it.”
The method is detailed in Nature Energy.
Additional coauthors from Rice, King Abdullah University of Science and Technology, Harvard University; and Northeastern University contributed to the work. Rice and the US Department of Energy Office of Science User Facilities supported the research.
Source: Rice University