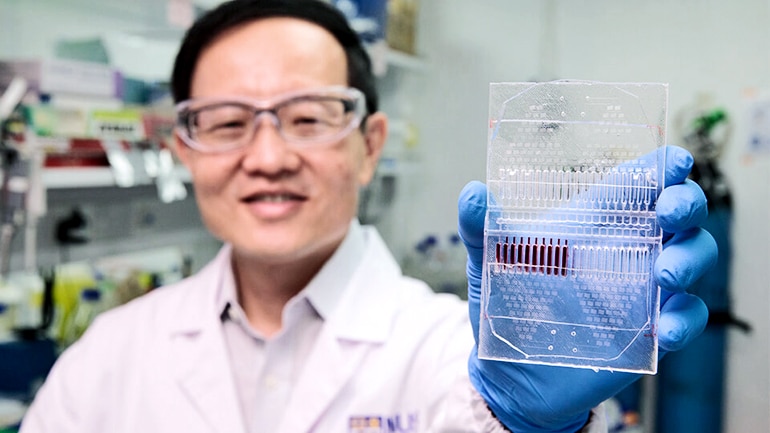
A new cancer cell-based assay could help doctors diagnose cancer, better monitor the disease, and take a step closer to customized treatment for individual patients.
The microfluidic device, which allows for precise control of fluids at the submillimeter scale, cultures circulating tumor cells (CTCs) collected from a patient’s blood and grows the CTC clusters in its microwells.
CTCs are cells that break away from the primary tumor and are carried around the body in the blood circulatory system. Researchers can obtain them from a simple blood draw, also known as a liquid biopsy.
Researchers say that assessing the CTCs can provide information about a patient’s specific cancer, without a highly invasive and painful tumor tissue biopsy. As these tumor cell clusters can closely mimic a patient’s tumor, different anticancer drugs can be tested on the clusters to determine the most effective treatment for the patient.
Liquid biopsy, which involves scanning the blood for CTCs, is the new wave in cancer screening. The assessment of CTCs can provide real-time information about a patient’s cancer and liquid biopsies can substitute current methods for detection and evaluation of cancer.
“Imaging techniques suffer from limitations in resolution that can lead to false-negative results. Tumor biopsies involve highly invasive procedures that can cause great discomfort and can also be expensive. Hence, tissue biopsies are generally used as a diagnostic tool only before and after cancer treatment,” says Lim Chwee Teck, principal investigator at the Mechanobiology Institute, Singapore and the biomedical engineering department at the National University of Singapore.
“In contrast, the evaluation of CTCs from liquid biopsies can provide regular, ongoing information for assessing metastatic risk, prognosis, and treatment efficacy.”
CTCs comprise many subpopulations and are very difficult to detect. So researchers must expand them before using them for clinical analysis. Conventional CTC expansion techniques take about six months or longer.
The new microfluidic device promotes CTC cluster formation within two weeks, with an overall cluster formation success rate of over 50 percent which is twice as high as current methods, which means patients can receive screening results faster.
The device could allow doctors to test a range of drugs on the cultured tumor cell clusters to see which would most effectively attack the cancer cells. The device also allows for the testing of two or more drugs, at various concentrations, at the same time, and could lead to the development of personalized therapies.
“Doctors are increasingly aware that a ‘trial and error’ or ‘one size fits all’ approach is not suitable for cancer treatment. This practice is inefficient and frequently results in inappropriate therapy and problematic side effects,” Lim says.
“In contrast, personalized treatment, tailored to the individual patient’s cancer type and progression, has the potential to increase efficacy and decrease toxicity. A critical advantage of our approach is its potential to predict a patient’s response to therapeutic treatment by performing tests on their own cancer cells.”
The findings appear in Nature Protocols.
“We are excited that this novel approach has the potential for translation into a hospital setting as prior approaches for growing cancer cells had low efficiency, required extensive periods for culture establishment, or compromised quality of the cells due to pre-enrichment,” says Lee Soo Chin from the National University Cancer Institute, Singapore.
‘Tractor beam’ sensor could find cancer in blood
“This device will provide a cost-effective and less-invasive means of routine monitoring of disease progression. The CTCs can be collected at various time points to determine which treatment would be most beneficial for the patient.”
Researchers are currently testing the assay on patient derived breast cancer cells and will extend the testing to other cancer types such as lung cancer. They have filed a patent for the assay and are looking into possible commercialization following clinical tests.
Source: National University of Singapore