Bacteria play a role in cloud formation, researchers report.
Meteorologists have known for almost 50 years that the proverbial flapping of a butterfly’s wings can trigger a hurricane in a completely different location.
The chaos theorist Edward Norton Lorenz coined the term “butterfly effect” in 1972 to describe the understanding that minimal changes in initial conditions can have a large effect on the later development of dynamic systems.
Results from the new research suggest that, in the future, meteorologists will have to pay attention not only to butterflies but also, and above all, to bacteria living in oceans.
“We have shown the circumstances under which these bacteria release a gas that plays a central role in the formation of clouds,” says Roman Stocker of the Institute of Environmental Engineering at ETH Zurich.
In their work in Nature Communications, the researchers looked at the microorganisms that feed on the metabolic products of marine phytoplankton. This term encompasses a wide variety of microscopic algae that together perform more photosynthesis than all plants. That means the true lungs of the Earth are not the forests, but the oceans: they produce about half the oxygen in the Earth’s atmosphere. Each year the phytoplankton also produce over 1.1 billion tons of a substance called dimethylsulphoniopropionate, or DMSP for short.
“DMSP satisfies 95% of marine bacteria’s sulphur demand and 15% of bacterial carbon demand,” says lead author Cherry Gao, a doctoral student in Stocker’s group.
To convert DMSP into biomass, the bacteria have two different metabolic pathways: if they demethylate it, they use both the sulphur and the carbon; if, however, they cleave it into several small molecules, they use only the carbon—while the sulphur escapes into the atmosphere in the form of dimethyl sulphide (DMS).
“DMS is what’s responsible for the typical smell of the sea,” Stocker says. In addition, DMS plays a pivotal role in cloud formation as a source of cloud condensation nuclei around which water vapor can condense.
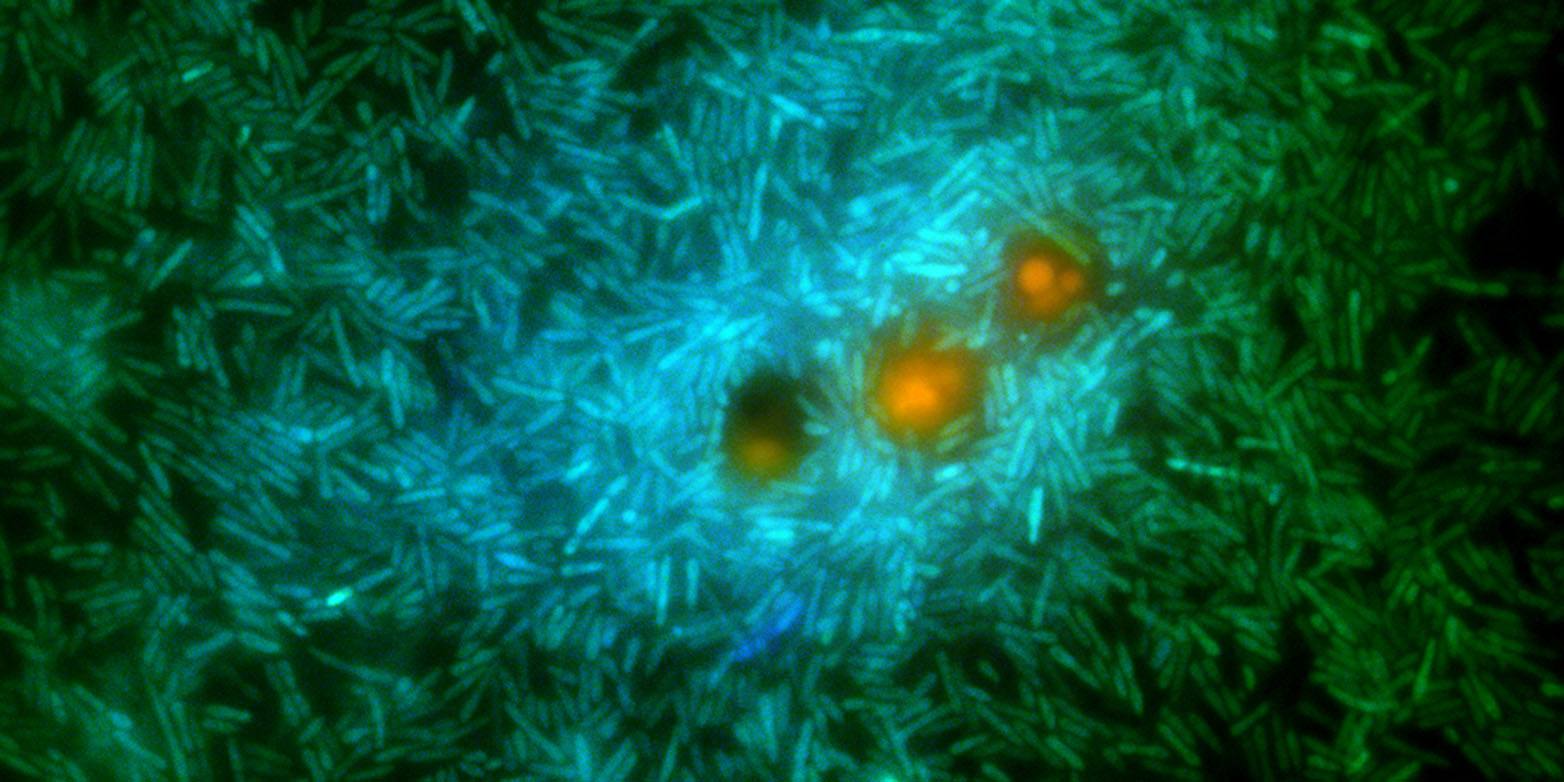
Until now, scientists did not understand what drove the bacteria to opt for one metabolic pathway or the other. Stocker’s research team genetically modified a marine bacterium of the species Ruegeria pomeroyi so that it fluoresced in different colors depending on the biochemical process it used to transform the DMSP. This enabled the researchers to show that at low concentrations of DMSP, the bacteria rely primarily on demethylation—while at high concentrations of a few micromoles per liter (about a quarter of a gallon), the cleavage process dominates.
The average concentration of DMSP in seawater is only a few nanomoles per liter. Under these circumstances, the metabolic pathway of cleavage is of negligible importance; the bacteria use the sulphur for their growth and cloud formation does not take place. “But the average—which is to say the concentration of DMSP found in a large bucket simply dunked into the sea in the conventional measurement method—tells only half the story,” Stocker says: “The other half reveals itself only on closer inspection.”
Because wherever phytoplankton blooms, DMSP concentrations can be thousands of times higher. It seems that marine bacteria have adapted to this unequal distribution of DMSP in seawater. If they grow in the direct vicinity of the microscopic algae, they start to cleave the DMSP.
“So the extent of cloud formation may ultimately also depend on the details of the interaction of algae and bacteria in the sea,” Stocker says.
Source: ETH Zurich



