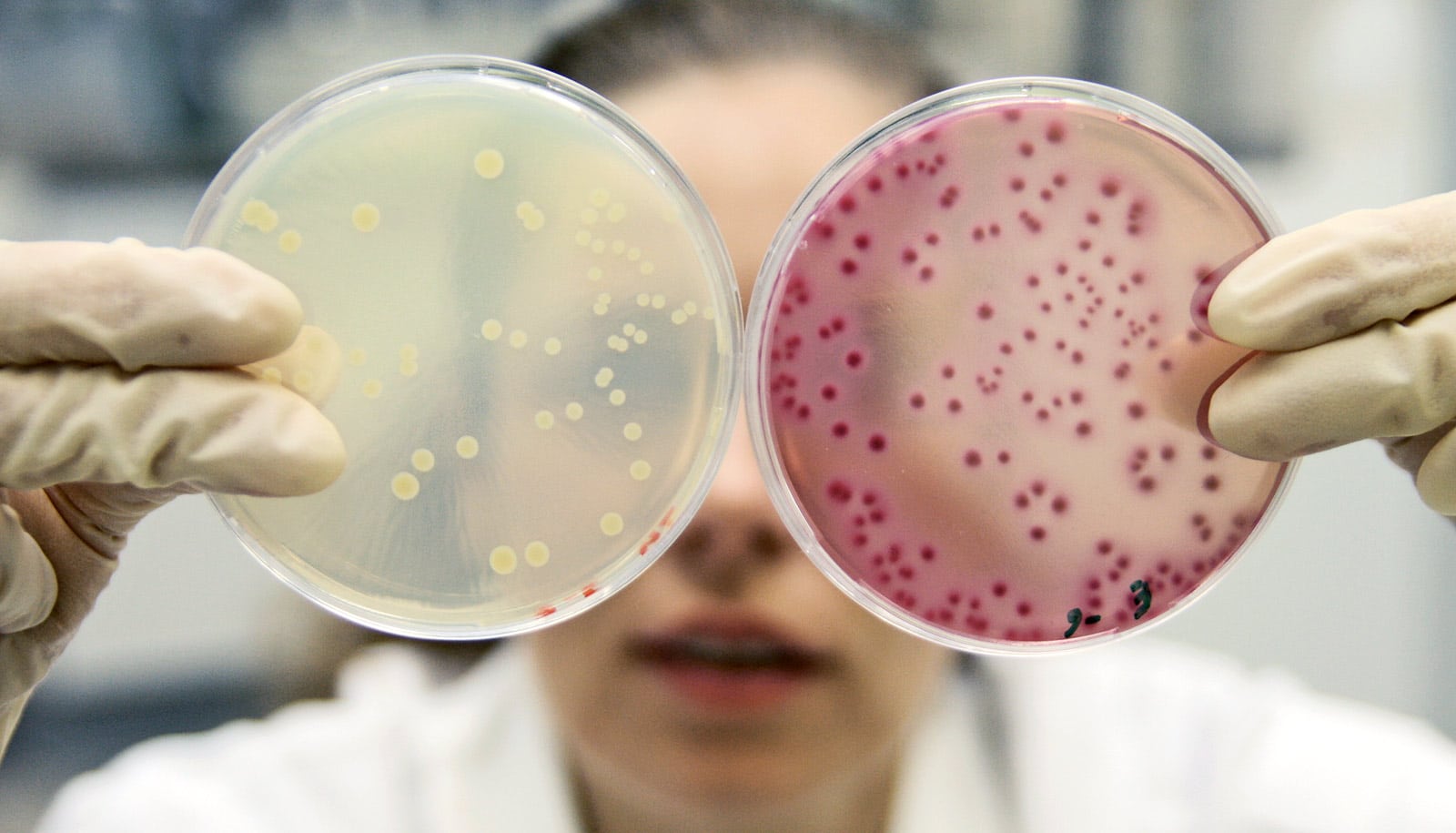
The bacterium Pseudomonas aeruginosa can produce specific molecular factors that dramatically increase or decrease the antibiotic resistance of Staphylococcus aureus, another bacterium that often co-infects with P. aeruginosa, researchers report.
“The interactions with P. aeruginosa can completely change S. aureus‘s susceptibility to standard antibiotics…”
The findings, which appear in a paper in PLOS Biology, point to the possibility of new antibiotics employing these factors to enhance antibiotic susceptibility.
The research also shows how understanding the precise mix of bacteria and their interactions could become a standard part of clinical practice in treating bacterial infections, especially the more dangerous infections involving antibiotic resistance. Doctors currently gauge the antibiotic susceptibility of an infecting bacterial species by examining it in isolation from other species.
The fight against antibiotic resistance
“The interactions with P. aeruginosa can completely change S. aureus‘s susceptibility to standard antibiotics,” says study senior author Brian P. Conlon, assistant professor of microbiology and immunology at the University of North Carolina at Chapel Hill.
Resistance to antibiotics by bacteria and other microbes is an ongoing public health crisis, contributing to about two million infections and 23,000 deaths per year in the United States, according to the Centers for Disease Control and Prevention.
P. aeruginosa, for instance, is a multidrug-resistant pathogen associated with hospital-acquired infections, including ventilator-associated pneumonia. As for S. aureus, some strains do not cause disease. Others cause the classic “staph” infections that antibiotics do kill. Other strains, though, are antibiotic-resistant.
Researchers have been racing to find ways to overcome the resistance of these and other bacteria.
One clue in the race to overcome antibiotic resistance that Conlon and colleagues uncovered is that S. aureus sometimes adopts a slow-growing, “low-energy” state that makes it more difficult to kill with antibiotics. Conlon’s team hypothesized that this low-energy state might arise from inter-species competition.
In other words, a co-infecting bacterial species might have evolved the capability to produce factors that put microbial competitors at a disadvantage. Such factors may include toxins, enzymes, or various bacterial components unique to specific strains.
“We know that P. aeruginosa commonly co-infects with S. aureus and secretes factors that mess with S. aureus‘s metabolism,” Conlon says. “So our hypothesis was that this interaction might be throwing S. aureus into a more antibiotic-resistant state.”
Conlon and colleagues, including first author Lauren Radlinski, a graduate student in the Conlon Laboratory who performed most of the experiments, investigated this possibility in the new study.
They set up a panel of S. aureus cultures, exposed them to molecules secreted by 14 different P. aeruginosa strains, and then tested the susceptibility of each culture to one of three antibiotics: vancomycin, tobramycin, and ciprofloxacin. The results were striking and have implications for clinical practice.
Changing vulnerability
The P. aeruginosa factors affected S. aureus‘s susceptibility to all three antibiotics, in some cases to an enormous extent. Some strains of P. aeruginosa, as expected, significantly reduced S. aureus‘s susceptibility to tobramycin and ciprofloxacin. Surprisingly, though, many other strains of P. aeruginosa greatly enhanced S. aureus‘s susceptibility to antibiotics used in the experiments.
Bacteria hitch rides on flies like ‘airborne shuttles’
“Factors secreted by eight of the P. aeruginosa strains, for example, induced 100 to 1,000 times more killing of S. aureus by vancomycin, compared to the control culture of S. aureus that was not exposed to P. aeruginosa factors,” Conlon says.
The researchers identified three specific P. aeruginosa factors that accounted for these effects:
- A protein-cutting enzyme called LasA increased vancomycin’s ability to kill S. aureus.
- A set of fat-related molecules called rhamnolipids increased S. aureus‘s uptake of tobramycin.
- A small organic molecule called HQNO inhibited the metabolism of S. aureus, shifting it into the low-energy state that made it more antibiotic-resistant.
Conlon and colleagues say it could be possible to create new antibiotics that include the susceptibility-enhancing factors LasA and rhamnolipids—and/or block the susceptibility-reducing factor HQNO—to build a better arsenal against serious bacterial infections.
Another approach would be to develop simple bacterial genetic tests that enable doctors to detect when a co-infecting bacterium is likely secreting factors that significantly influence antibiotic susceptibility.
Swabbing dolphin mouths reveals bacterial ‘dark matter’
Conlon’s team is now sequencing P. aeruginosa strains to see how gene sequences vary between strains and how this variance affects the ability of these strains to produce the aforementioned factors Conlon’s lab has described.
The National Institute of Allergy and Infectious Diseases (NIAID) funded this research.
Source: UNC- Chapel Hill