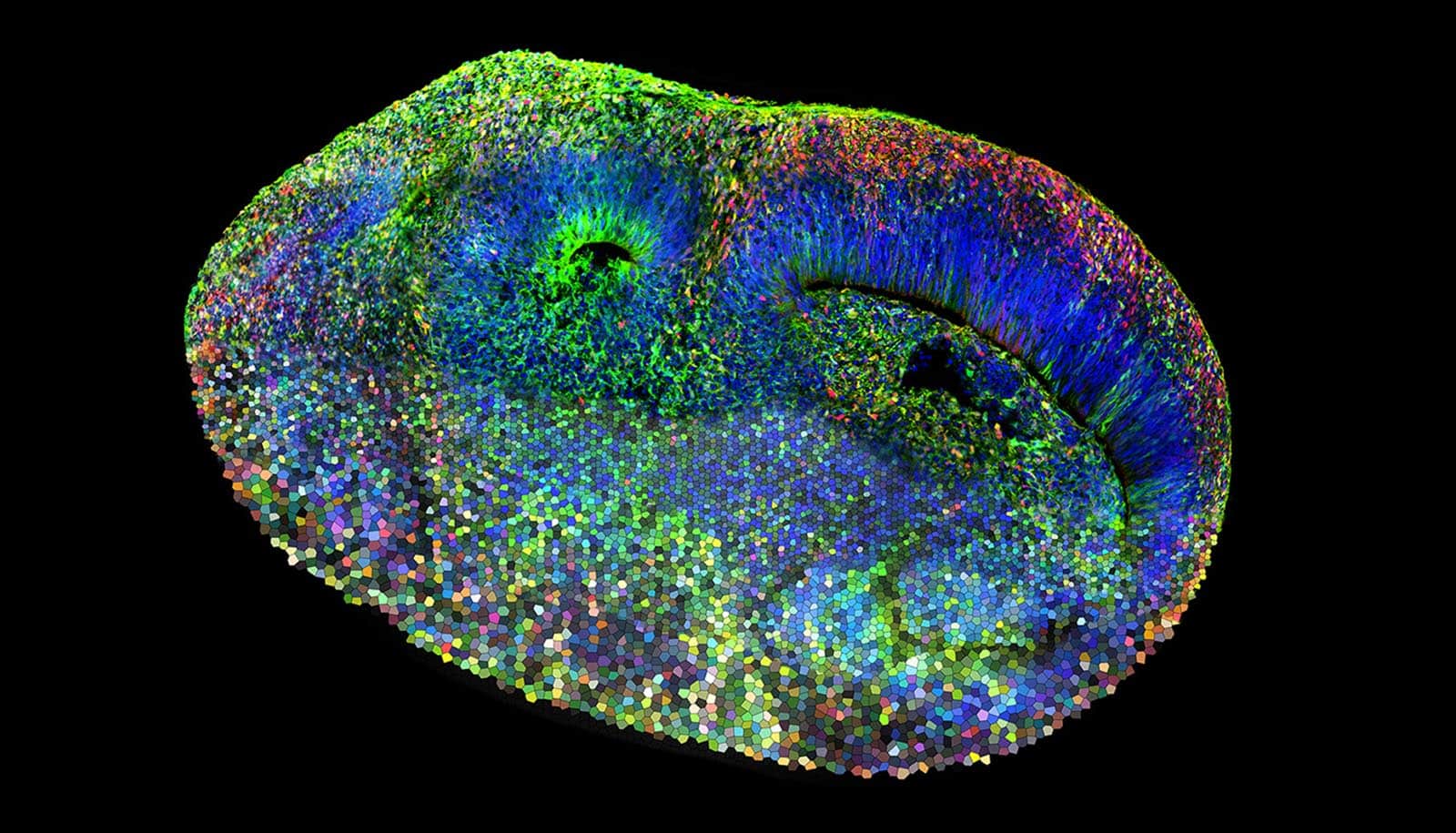
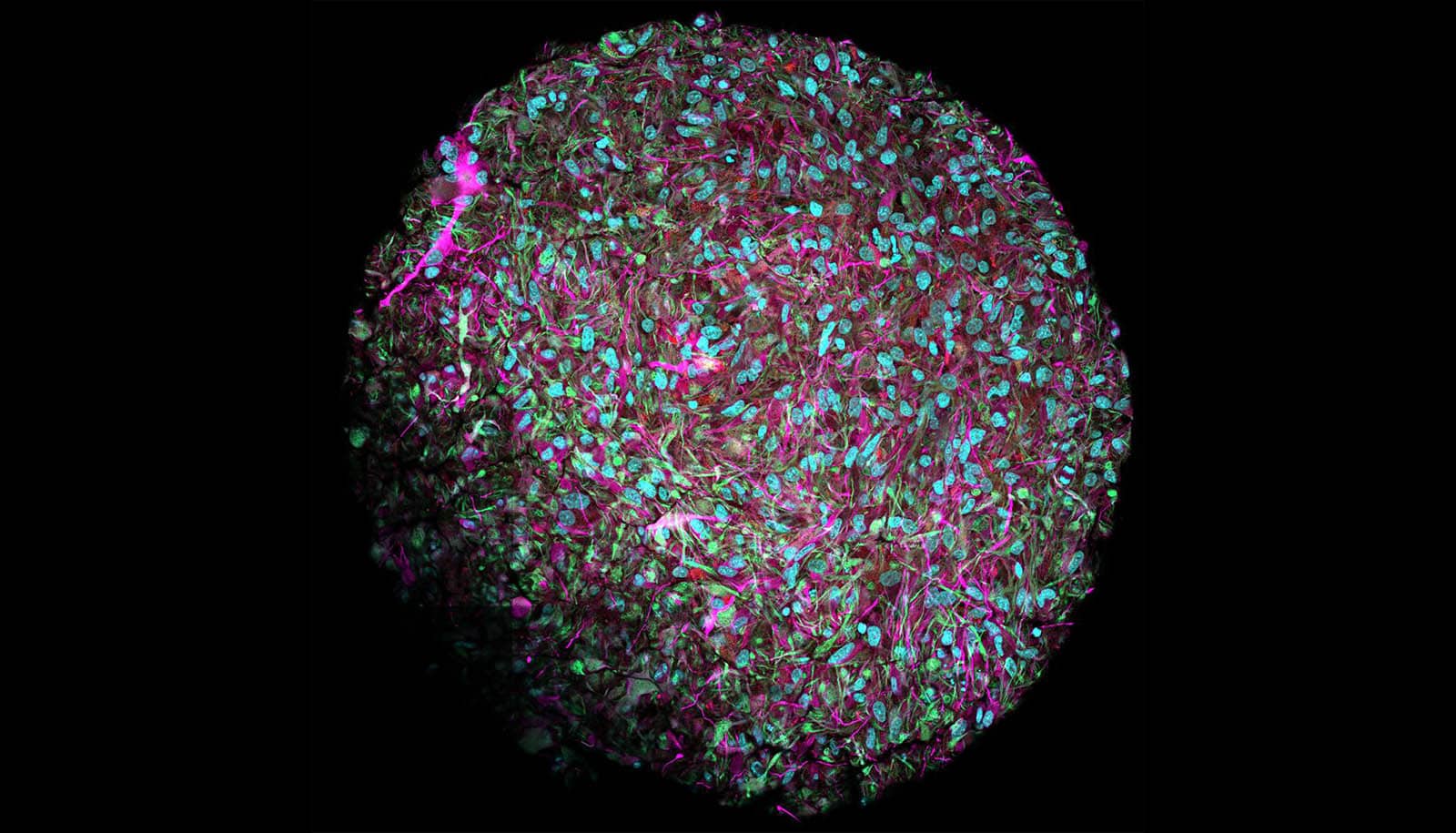
A new brain organoid model lets researchers investigate the origins of autism.
Organoids are microtissue spheroids that are grown from stem cells and have a similar structure to real organs.
With them, and with a new method for modifying genes within these organoids using the CRISPR-Cas gene scissors, the researchers found out which genetic networks in which cell types in the brain are responsible for the development of autism.
“Our model offers unparalleled insight into one of the most complex disorders affecting the human brain and brings some much needed hope to clinical autism research,” says Jürgen Knoblich, professor and scientific director of the Institute of Molecular Biotechnology (IMBA) of the Austrian Academy of Sciences in Vienna and coauthor of the study in Nature.
This new method enabled the researchers to genetically modify the cells of a brain organoid in a mosaic-like fashion and then study them systematically. Specifically, the scientists altered one of 36 different genes associated with autism in each of the individual cells and studied the resulting effects. “We can see the consequences of every mutation in a single experiment, thus reducing the analysis time drastically when compared to conventional methods,” Knoblich says.
The new method was developed by researchers at the IMBA in Vienna, based on previous methods. In the current study, the group led by Barbara Treutlein, professor of quantitative developmental biology at ETH Zurich, incorporated the computer-assisted analysis of the raw research data: changing multiple genes in parallel in a human organoid and analyzing the resulting effects at the level of thousands of individual cells creates a vast amount of data.
In human disease research, organoids offer advantages over research using lab animals: unlike in lab animals, human genes and cells can be studied in organoids. These advantages are particularly significant in neuroscience, as the specific processes responsible for the development of the human cerebral cortex are unique to the human brain.
Neurodevelopmental disorders in humans are due in part to these human-specific processes in brain development. For example, many human genes that confer an increased risk for an autism spectrum disorder are genes that are critical for cortex development.
Previous studies have shown a causal link between certain gene mutations and autism. However, scientists still don’t understand how these mutations lead to defects in brain development and the expression of autism spectrum disorders. Due to the uniqueness of human brain development, the utility of animal models in this case is limited. “Only a human model of the brain like the one we used can reproduce the complexity and particularities of the human brain,” Knoblich says.
In their organoid model, the researchers were able to show that the genetic changes that are typical for autism affected mainly certain types of neural precursor cells. These are the founder cells from which neurons are created. “This suggests that molecular changes occur at an early stage in fetal brain development that ultimately lead to autism,” says Chong Li, a postdoc at IMBA and one of the study’s two lead authors. “Some cell types we identified are more vulnerable in autism and should receive more attention in future research.”
In addition, the scientists reveal that there are connections among the 36 genes they studied that confer a high risk for autism: “Using a program we developed, we were able to show that these genes are connected to each other through a gene regulation network, and that they interact with each other and have similar effects in the cells,” says Jonas Fleck, a doctoral student in Treutlein’s group and the other lead author of the study.
To test whether the results from the organoid model actually apply to autistic people, the researchers teamed up with clinicians at the Medical University of Vienna to produce brain organoids from two stem cell samples from affected individuals. They found that the organoid data closely matched clinical observations in the affected individuals.
The researchers emphasize that their technology for changing organoid cells in a mosaic-like fashion can also be used for organoids of other human organs and to study other diseases. This is a new research tool for rapidly examining a large number of disease-associated genes. “This technology thus helps to obtain relevant research results directly with human organoids in cell culture,” Treutlein says. “What’s more, human organoid disease models can also be used to test drug efficacy, which can help reduce animal testing.”
Source: ETH Zurich via IMBA