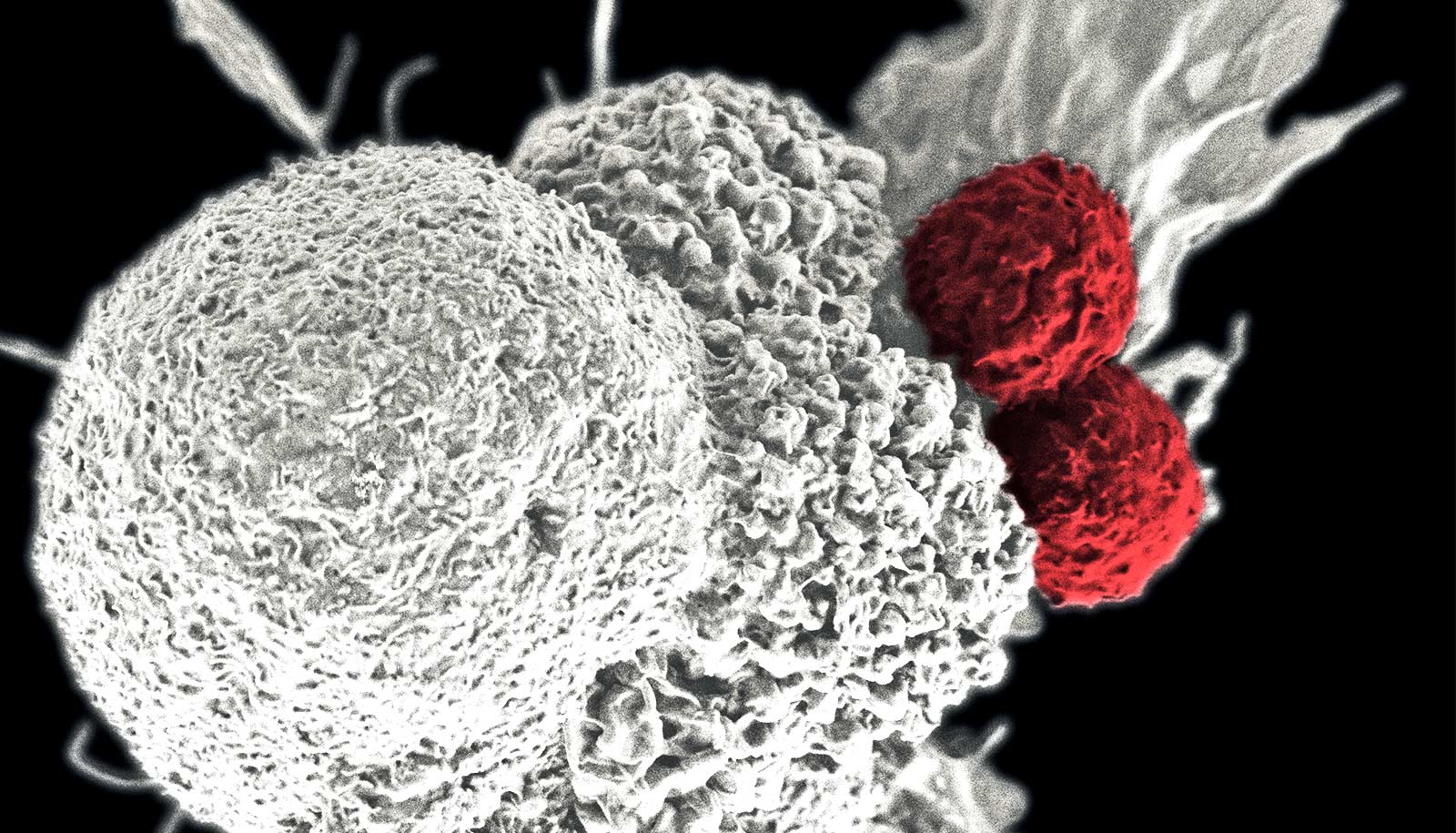
Scientists have developed an artificial lymph node with the potential to treat cancer, according to a new study in mice and human cells.
The newly developed lymph node—a sac filled with immune system components—is implanted under the skin. It is designed to act like a learning hub and stimulator to teach immune system T cells to recognize and kill cancer cells.
Lymph nodes—tiny glands throughout the body, mainly in the neck, armpits, and groin—are part of the immune systems of mammals, including mice and people. They number in the hundreds so that immune cells in one area of the body don’t have to travel far to alert the immune system to impending danger.
“They are a landing spot where T cells, the immune system’s fighting cells, lay dormant, waiting to be activated to fight infections or other abnormal cells,” says Natalie Livingston, currently a postdoctoral researcher at Massachusetts General Hospital and first author of the study published in the journal Advanced Materials.
“Because cancers can trick T cells into staying dormant, the artificial lymph node was designed to inform and activate T cells that are injected alongside the lymph node.”
To create the artificial lymph node, the scientists used hyaluronic acid, a moisturizing substance commonly used in cosmetics and lotions and found naturally in the body’s skin and joints.
Because of its properties, hyaluronic acid is often used in biodegradable materials such as wound healing patches meant to be implanted or applied to the body. Among those properties, hyaluronic acid can connect with T cells via a cell surface receptor.
For the current study, the team used hyaluronic acid as the scaffolding, or base, for their new lymph node, and added MHC (major histocompatibility complex) or HLA (human histocompatibility antigen) molecules, which rev up T cells and other immune system components. Then, they also added molecules and antigens common to cancer cells to “teach” T cells what to look for.
“By adding different antibodies to the artificial lymph node, we have the ability to control what the T cells are being activated to search for,” Livingston says.
The resulting artificial lymph node is about 150 microns in size, about twice the width of a human hair. It’s small enough to remain under the skin and large enough to avoid being swept away in the blood stream.
“An advantage to this approach over other cell-based therapies such as CAR-T is fewer manufacturing steps,” says Jonathan Schneck, a professor of pathology, medicine, and oncology at the Johns Hopkins University School of Medicine, director of the Johns Hopkins Center for Translational Immunoengineering, and a member of the Institute for Cell Engineering, Kimmel Cancer Center, and Institute for Nanobiotechnology.
Current cell-based therapies require extracting T cells from a patient, manipulating them outside of the body to recognize a particular type of cancer, and injecting them back into the patient.
“In our approach, we inject T cells along with an artificial lymph node, and the T cells get primed and educated by the artificial lymph node inside of the body. Then, the T cells can travel anywhere to destroy cancer cells,” says Schneck, who led the research team, along with Hai-Quan Mao, director of Johns Hopkins’ Nanobiotechnology Institute.
Livingston, Schneck, and colleagues tested the artificial lymph node in mice that were implanted with either melanoma or colon cancers. Six days after the tumors were implanted, the mice received injections of the artificial lymph node and T cells.
The researchers compared these mice with ones receiving the artificial lymph node alone, those receiving T cells alone (which have not been activated by the artificial lymph node) and those receiving T cells in combination with a class of immunotherapy drug called anti-PD-1.
Nine days later, mice with melanomas and colon cancers that received a combination of the artificial lymph node, T cells, and the anti-PD-1 drug had the best survival rates (three of the seven mice were still alive at 33 days), compared with other groups that only lived to about 26 days. This group of mice also had the slowest cancer growth rate. It took between five and 10 days longer for their cancers to double in size than the other groups.
The scientists also found that the artificial lymph node attracted an influx of other immune cells and acts as an “immunologically active niche” to help to further stimulate the immune system. When T cells were injected into the mice alongside the artificial lymph node, T cell numbers grew as much as nine times more plentiful.
Livingston says the artificial lymph node approach is different from a cancer vaccine, which typically activates a dendritic cell, an immune system component that teaches T cells what to search for. People with cancer often develop malfunctioning dendritic cells, and the artificial lymph node skips the dendritic cell to directly activate T cells.
The research team plans to conduct additional laboratory studies to add more immune signaling molecules to the lymph node and recruit more of the host’s immune cells to the artificial lymph node environment.
“We blended the disciplines of materials science and immunology to create a potential therapy that forms its own immunology community—a kind of living drug,” says Schneck.
The researchers have filed for a patent involving the technology described in their research.
The National Institutes of Health, the National Science Foundation, the Ruth L. Kirschstein Predoctoral Individual National Research Service Award, the National Science Foundation Graduate Research Fellowship, the ARCS Foundation, the Siebel Foundation, and the Natural Sciences and Engineering Research Council of Canada’s Postgraduate Scholarships—Doctoral Award funded the work.
Source: Johns Hopkins University