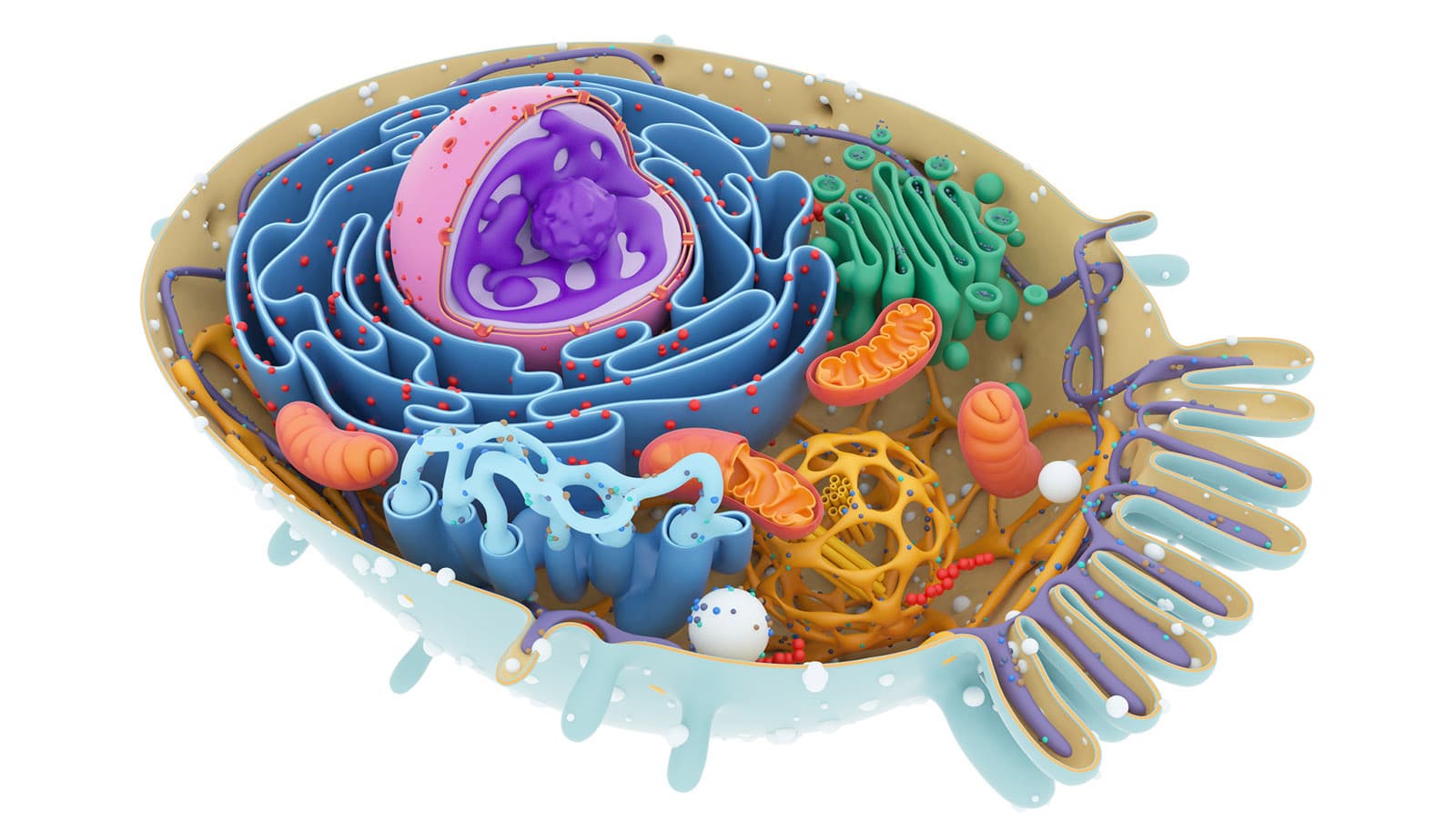
Scientists have long understood that parts of cells, called organelles, evolved to have certain shapes and sizes because their forms are closely related to how they function. Now, researchers have developed a bacteria-based tool to test whether, as the axiom goes, form follows function.
The tool, which researchers say may someday have practical applications in treating illness, works by precisely targeting and dismantling the outer membrane surrounding organelles, and is being made freely available to other scientists. In an interesting twist, say the researchers, the tool may also be able to dismantle aggregated proteins in cells that often characterize neurodegenerative conditions, such as amyotrophic lateral sclerosis (ALS).
The team focused their work on mitochondria, organelles that serve as the energy engines or powerhouses of cells, including human ones. They also focused on so-called Golgi bodies that act as factories and packagers of a variety of proteins and the nucleus, or control center of a cell.
Results of the researchers’ work appears in Cell Reports.
“We developed a scientific tool to test why cell organelles look the way they do in order to have a certain function,” says Takanari Inoue, a professor of cell biology at the Johns Hopkins University School of Medicine. The tool, says Inoue, may also help reveal why function may change—for better or worse—when an organelle’s shape is different.
In the case of mitochondria, for example, in people with Alzheimer’s disease, they enlarge and become disorganized inside, according to Inoue. In people with an accelerated aging disease called progeria, the nucleus is misshapen.
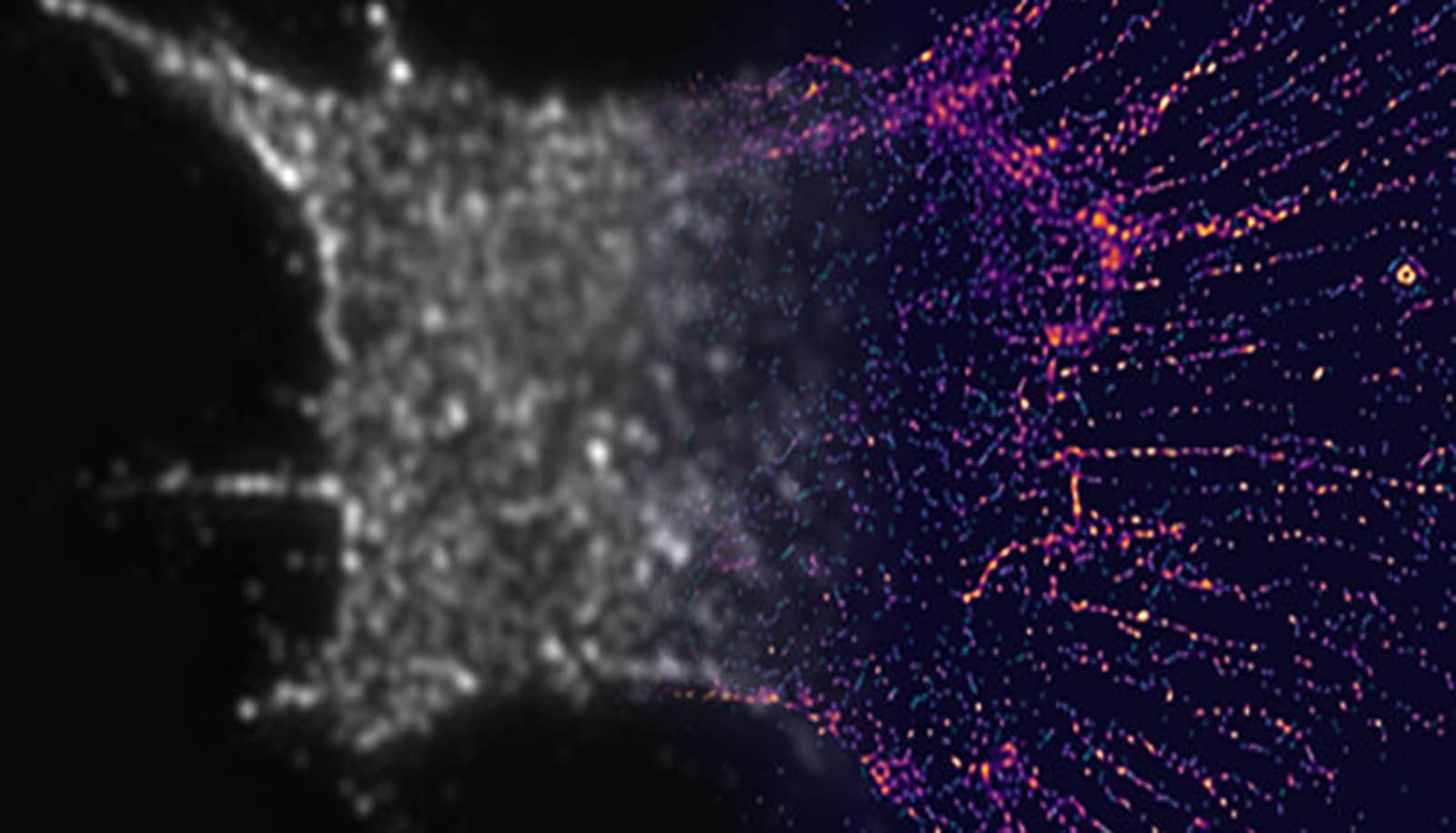
To develop the tool, a postdoctoral researcher in Inoue’s laboratory, Hideki Nakamura, recruited Listeria bacteria, which, because of their particular effects, cause food-borne illnesses. When Listeria invade an animal cell, they hijack the cell’s stores of actin, a protein that helps them move through the cell, where they soak up nutrients and find a way to escape and infect other cells.
Working with physics experts who can identify and measure the physical force generated by Listeria’s seizing of actin, Nakamura engineered the Listeria-linked actin to assemble proteins and other molecules that connect with the surface of an organelle within a cell, exert force on the surface, and break it open.
Scientists already have other methods to break open organelles inside cells, says Inoue, such as using so-called optical tweezers or stretching out the cell to flatten it. However, those methods probe the cell from the outside, and none of them, he says, can target organelles from inside the cell.
Nakamura, who is now at Kyoto University, Inoue, and their team dubbed the new tool ActuAtor.
In their new set of experiments, the team tested ActuAtor on human epithelial cells that line and cover the surfaces of skin and other organs, and were able to completely fragment mitochondria in the cells 10 minutes after the Listeria-based tool entered the cells. When the team looked at mitochondrial function before and after the mitochondria’s shape was altered, they found no large differences in their ability to generate power for the cell but did find that the cell “recognizes” that the mitochondrial shape is different, and increases efforts to get rid of the misshapen organelles, albeit only slightly.
“In this case, our team concluded that function may not follow form in mitochondria,” says Inoue.
The team also tested the tool on brain cells and different organelles, including nuclei and Golgi bodies, and were able to use ActuAtor to break open the organelles.
Inoue and Nakamura further repurposed ActuAtor to disperse the accumulation of protein granules inside cells that form because of “environmental stress” such as changes in temperature or lack of oxygen. The team says they’ll test this application of the tool on its ability to disperse protein aggregates that clump in brain cells in efforts to treat neurodegenerative diseases such as ALS.
Funding for the research came from the Japanese Science and Technology Agency; the Japan Society for the Promotion of Science; the Mochida Memorial Foundation for Medical and Pharmaceutical Research; the National Institutes of Health; the Human Frontier Science Program; the Department of Defense; the Alfred P. Sloan, McKnight, Klingenstein and Simons, and Vallee awards; the Kavli Institute; and the Air Force Research Laboratory.
Source: Johns Hopkins University