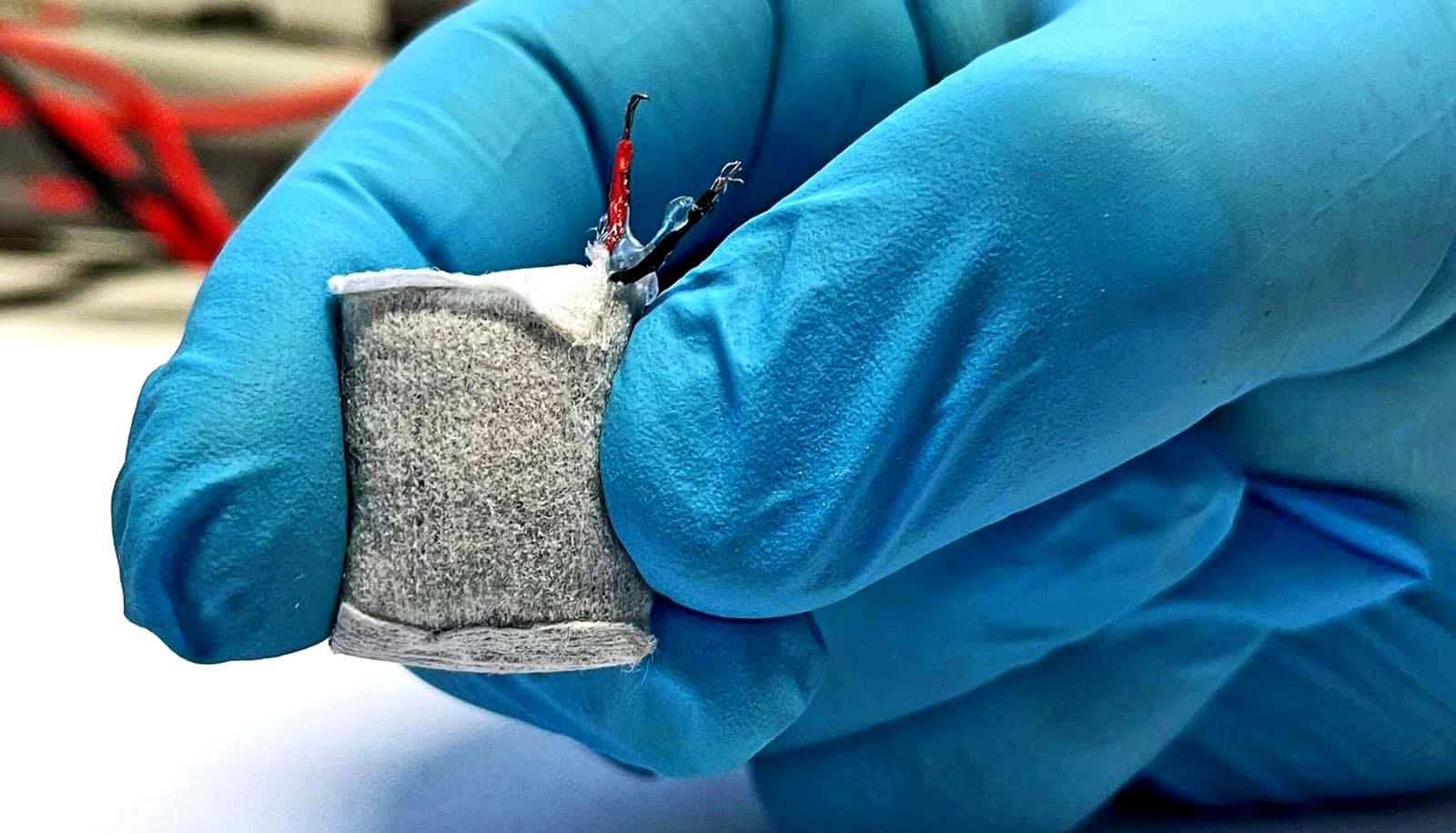
Researchers have developed an implantable fuel cell that uses excess blood sugar from tissue to generate electrical energy.
The researchers combined the fuel cell with artificial beta cells that produce insulin at the touch of a button and effectively lower blood glucose levels much like their natural role models in the pancreas.
In type 1 diabetes, the body does not produce insulin. This means that patients have to obtain the hormone externally to regulate their blood sugar levels. Nowadays, this is mostly done via insulin pumps that attach directly to the body. These devices, as well as other medical applications such as pacemakers, require a reliable energy supply, which primarily comes from single-use or rechargeable batteries.
“Many people, especially in the Western industrialized nations, consume more carbohydrates than they need in everyday life,” says Martin Fussenegger, professor of biotechnology and bioengineering at ETH Zurich. This, he adds, leads to obesity, diabetes, and cardiovascular disease. “This gave us the idea of using this excess metabolic energy to produce electricity to power biomedical devices,” he says.
“The new system autonomously regulates insulin and blood glucose levels and could be used to treat diabetes in the future.”
At the heart of the fuel cell is an anode (electrode) made of copper-based nanoparticles, which Fussenegger’s team created specifically for this application. It consists of copper-based nanoparticles and splits glucose into gluconic acid and a proton to generate electricity, which sets an electric circuit in motion.
Wrapped in a nonwoven fabric and coated with alginate, an algae product approved for medical use, the fuel cell resembles a small tea bag that can be implanted under the skin. The alginate soaks up body fluid and allows glucose to pass from the tissue into the fuel cell within.
In a second step, the researchers coupled the fuel cell with a capsule containing artificial beta cells. These can be stimulated to produce and secrete insulin using electric current or blue LED light. Fussenegger and his colleagues already tested such designer cells some time ago.
The system combines sustained power generation and controlled insulin delivery. As soon as the fuel cell registers excess glucose, it starts to generate power. This electrical energy is then used to stimulate the cells to produce and release insulin into the blood. As a result, blood sugar dips to a normal level. Once it falls below a certain threshold value, the production of electricity and insulin stops.
The electrical energy provided by the fuel cell is sufficient not only to stimulate the designer cells but also to enable the implanted system to communicate with external devices such as a smartphone. This allows potential users to adjust the system via a corresponding app. A doctor could also access it remotely and make adjustments.
“The new system autonomously regulates insulin and blood glucose levels and could be used to treat diabetes in the future,” Fussenegger says.
The existing system is only a prototype. Although the researchers have successfully tested it in mice, they are unable to develop it into a marketable product. “Bringing such a device to market is far beyond our financial and human resources,” Fussenegger says. This would call for an industry partner with the appropriate resources and know-how.
The study appears in Advanced Materials.
Source: ETH Zurich