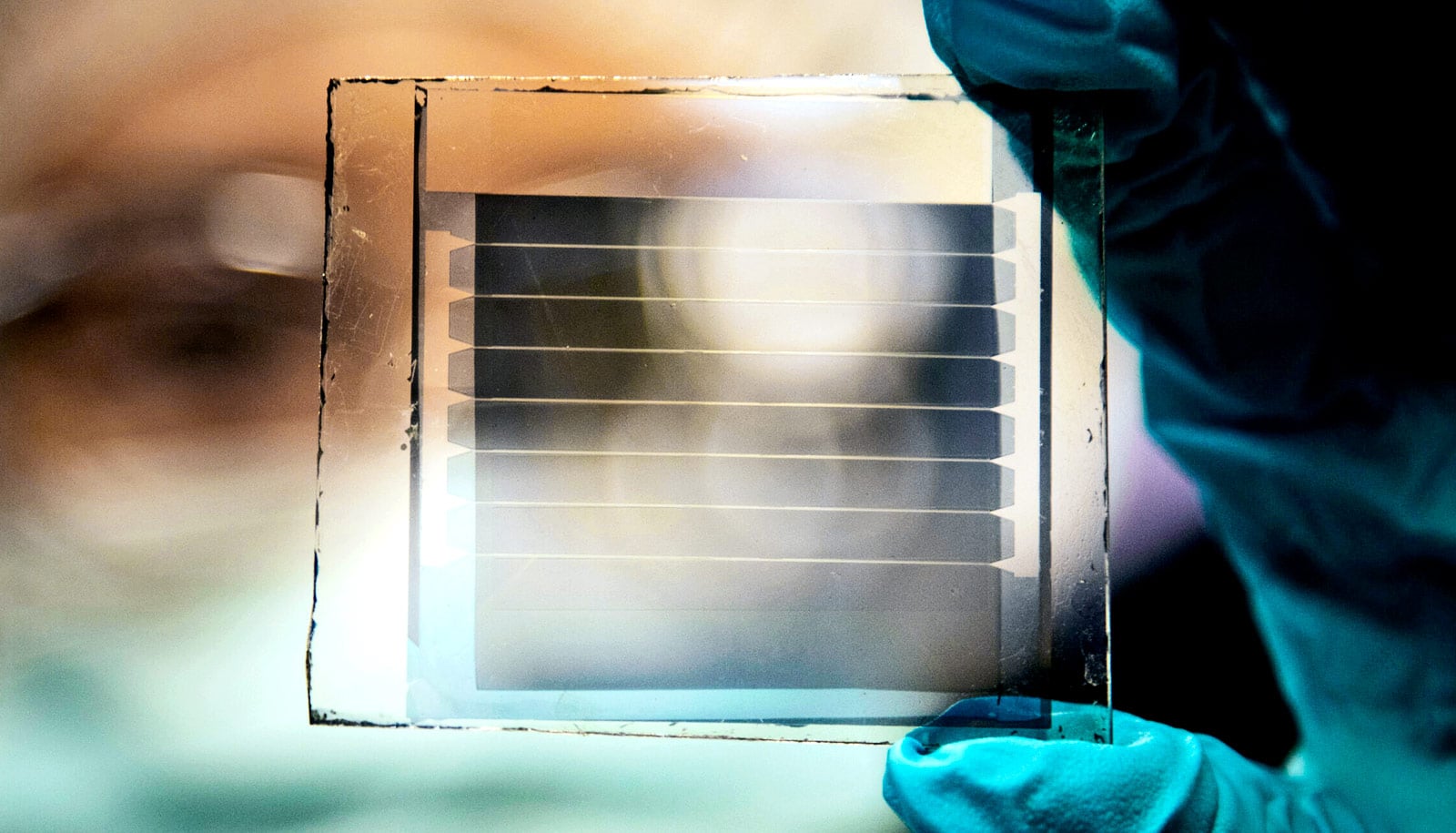
A new transparency-friendly solar cell design could marry high efficiencies with 30-year estimated lifetimes, researchers report.
It may pave the way for windows that also provide solar power.
“Solar energy is about the cheapest form of energy that mankind has ever produced since the industrial revolution,” says Stephen Forrest, a professor of electrical engineering at the University of Michigan, who led the research. “With these devices used on windows, your building becomes a power plant.”
While silicon remains king for solar panel efficiency, it isn’t transparent. For window-friendly solar panels, researchers have been exploring organic—or carbon-based—materials. The challenge for Forrest’s team was how to prevent very efficient organic light-converting materials from degrading quickly during use.
The strength and the weakness of these materials lie in the molecules that transfer the photogenerated electrons to the electrodes, the entrance points to the circuit that either uses or stores the solar power. These materials are known generally as “non-fullerene acceptors” to set them apart from the more robust but less efficient “fullerene acceptors” made of nanoscale carbon mesh. Solar cells made with non-fullerene acceptors that incorporate sulfur can achieve silicon-rivaling efficiencies of 18%, but they do not last as long.
The researchers set out to change that. In their experiments, they showed that without protecting the sunlight-converting material, the efficiency fell to less than 40% of its initial value within 12 weeks under the equivalent of 1 sun’s illumination.
“Non-fullerene acceptors cause very high efficiency, but contain weak bonds that easily dissociate under high energy photons, especially the UV [ultraviolet] photons common in sunlight,” says Yongxi Li, assistant research scientist in electrical engineering and computer science and first author of the paper.
By studying the nature of the degradation in those unprotected solar cells, the team recognized that they only needed shoring up in a few places. First, they’d need to block out that UV light. For that, they added a layer of zinc oxide—a common sunscreen ingredient—on the sun-facing side of the glass.
A thinner zinc oxide layer next to the light absorbing region helps conduct the solar-generated electrons to the electrode. Unfortunately, it also breaks down the fragile light absorber, so the team added a layer of a carbon-based material called IC-SAM as a buffer.
In addition, the electrode that draws positively-charged “holes”—essentially spaces vacated by electrons—into the circuit can also react with the light absorber. To protect that flank, they added another buffer layer, this one a fullerene shaped like a soccer ball.
The team then tested their new defenses under different intensities of simulated sunlight, from the typical 1 sun up to the light of 27 suns, and temperatures up to 150 degrees Fahrenheit. By studying how the performance degraded under these conditions, the team extrapolated that the solar cells would still be running at 80% efficiency after 30 years.
Forrest sees a future of these devices “coming to a window near you.” His team has already increased the transparency of the module to 40%. They believe they can approach 60% transparency.
They’re also working on bumping up the efficiency from the 10% achieved in the reported semitransparent modules, closer to the 15% believed to be possible at high transparency. Because the materials can be prepared as liquids, the manufacturing costs are expected to be relatively low.
The research appears in Nature Communications. Additional researchers from North Carolina State University and Tianjin University and Zhejiang University in China contributed to the work.
Funding for the research came from the Office of Naval Research and the US Department of Energy’s Office of Energy Efficiency and Renewable Energy. Universal Display Corp. holds a license to the work. The University of Michigan has a financial interest and Forrest has an ownership interest in Universal Display Corp.
Source: University of Michigan