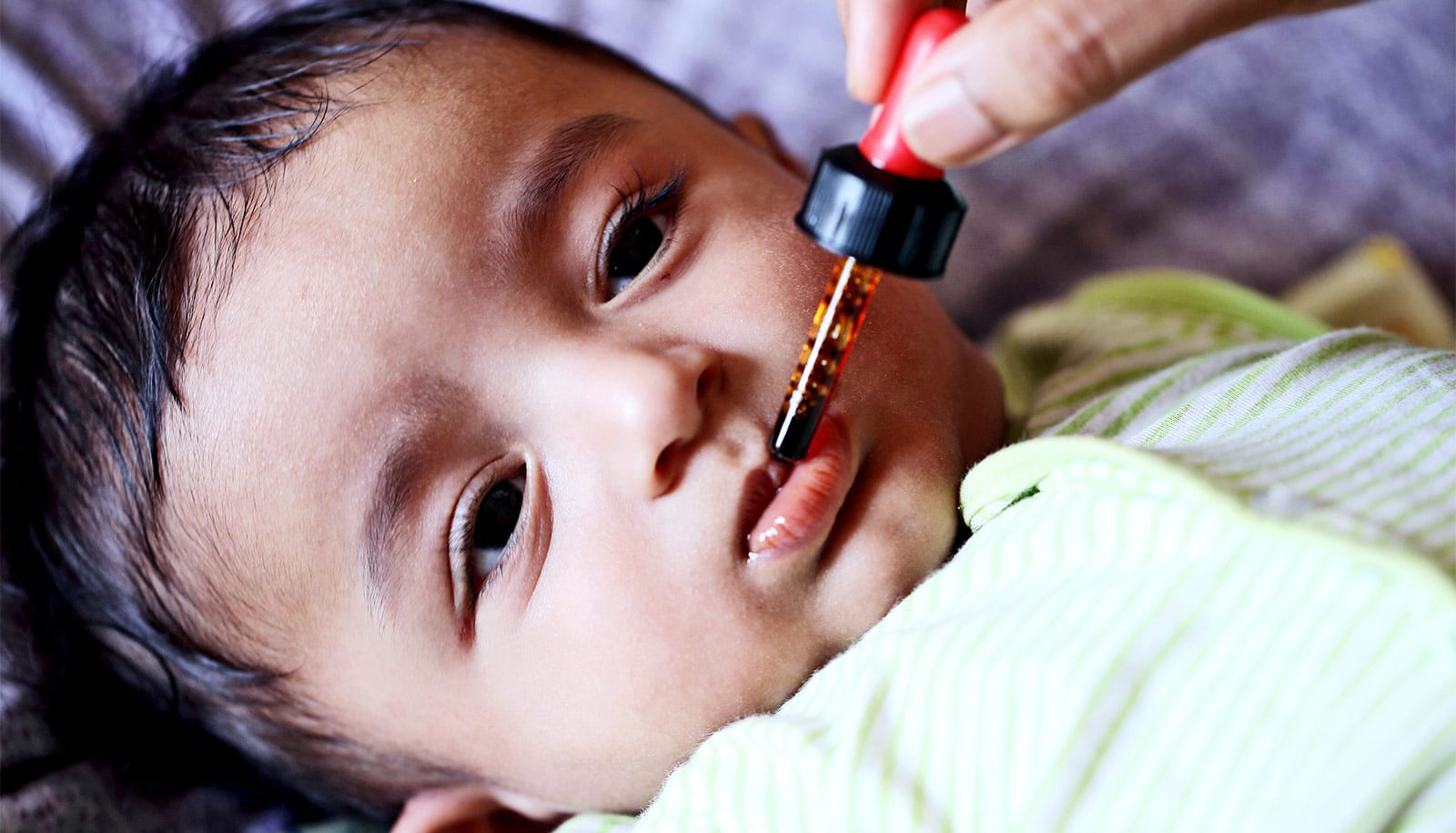
Environmental enteric dysfunction, a chronic gut disorder that occurs in regions with poor sanitation, disrupts intestinal immune responses and weakens the effectiveness of an oral vaccine in a mouse model of the disease.
Findings of the study in Immunity are important because oral vaccines delivered by liquid drops to the mouth, such as polio and rotavirus vaccines, are especially useful in low-income countries that may not have health care workers trained in administering vaccines through needles.
Oral vaccines may also stimulate better local immunity in the gut, which is key for fending off diseases contracted through contaminated food and water—including some of the very infections that contribute to environmental enteric dysfunction (EED).
“It is tragic that the exact vaccines that might help prevent EED don’t work in children who have the disease,” says senior author Timothy Hand, assistant professor of pediatrics and immunology at the R.K. Mellon Institute for Pediatric Research at the University of Pittsburgh Medical Center Children’s Hospital and director of the Gnotobiotic Animal Core Lab.
EED mice show stunted growth
Malnutrition and chronic gastrointestinal infection from contaminated food and water cause EED. Infection with viruses, parasites, or bacteria combined with poor diet can trigger gut inflammation and damage the finger-like projections called villi that help absorb nutrients from food.
“EED can affect anyone, but it’s a major problem in children because they’re still developing,” says Hand. “The result is that children with EED are stunted. They end up shorter in stature. But perhaps more importantly, it can significantly affect brain development: These children have less cognitive ability. And this is a lifelong problem; you can’t restore that development later in life.”
“If we could get flush toilets and plumbing to the world, we wouldn’t have this disease.”
To learn more about the mechanisms behind oral vaccine failure, Hand and his team developed a mouse model of the disease. To induce EED-like symptoms, they fed the rodents a diet deficient in fat and protein and inoculated them with a strain of E. coli bacteria that invades gut cells.
Like humans with the disease, EED mice had stunted growth, shifts in the gut microbiome composition, elevated gut inflammation, and shortened gut villi compared with control mice that received a normal diet with adequate fat and protein or animals that received a normal diet and bacteria or a poor diet without bacteria.
After giving the mice an oral vaccine, the researchers found that immune responses were severely compromised in those with EED. Vaccine-specific CD4+ T cells in the small intestine were about 18 times lower than in control mice.
Target the microbiome
Further experiments indicated that oral vaccine failure in EED mice was mediated by their gut microbiome. In response to microbiome-associated inflammation, T regulatory (Treg) cells accumulate in the small intestine of EED mice.
“Treg cells arise because there’s too much inflammation and they help tamp down that inflammation,” says Hand. “But unfortunately, a side effect is that they prevent local accumulation of vaccine-specific CD4+ T cells.”
When the team used antibiotics to eliminate gut bacteria, vaccine effectiveness was restored in EED mice. These findings support the idea that targeting the microbiome could help treat EED and improve vaccine success in children, Hand says.
“Judicious use of antibiotics in these children might be able to reset the small intestinal microbiome, reduce inflammation in the small intestine, and reduce those Tregs,” he says.
EED is rare in resource-rich countries but common in poorer countries that lack sewage systems and sanitation. About 150 million children worldwide live in conditions that put them at risk of getting the disease.
“If we could get flush toilets and plumbing to the world, we wouldn’t have this disease,” Hand says, “What’s causing these chronic infections is that people are either drinking contaminated water or flies are transporting diseases from sewage to food.”
In the future, Hand and his team plan to collaborate with researchers in countries where EED is a problem to better understand vaccine outcomes in children with this disease.
Additional coauthors on the research are from Tulane University, Central South University in China, the National Institutes of Health, and the University of Pittsburgh.
The National Institutes of Health, the R.K. Mellon Institute for Pediatric Research, and UPMC Children’s Hospital of Pittsburgh supported the work.
Source: University of Pittsburgh