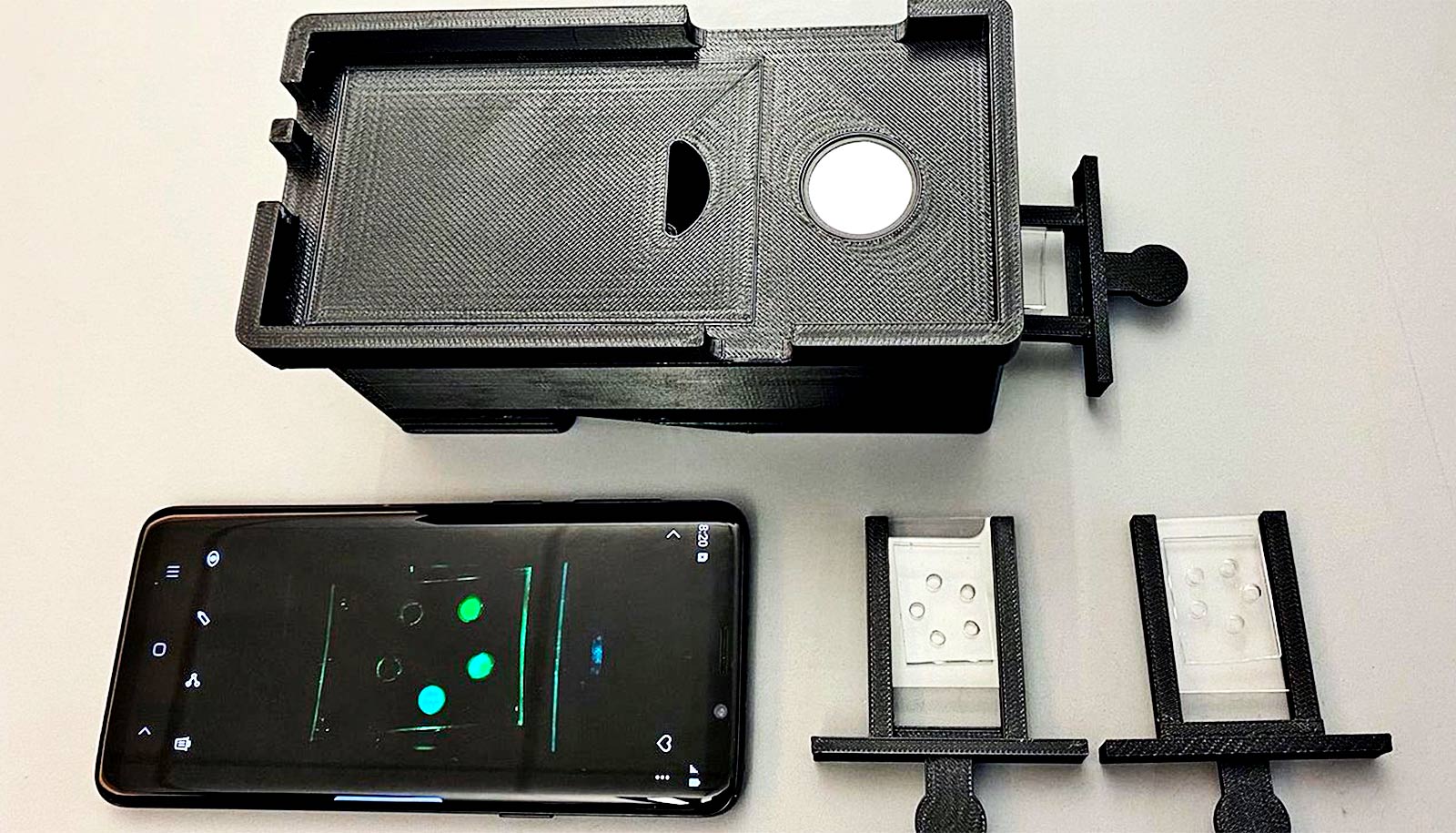
A smartphone-based device can read a new COVID-19 saliva test to get results in 15 minutes, researchers report.
The test address the need to expand testing capacity in community-based settings
The new test uses the same CRISPR-based approach that the researchers have submitted to the Food and Drug Administration for Emergency Use Authorization.
Rapid PCR tests typically use nasal swab samples and are performed in laboratory settings by highly trained individuals using sophisticated equipment. The new saliva-based COVID-19 test, which doesn’t require lab processing, could rapidly expand testing capacity in outpatient clinics, community testing sites, and other locations.
The assay the researchers developed detects SARS-CoV-2 virus RNA in saliva to diagnose COVID-19 and is more sensitive than PCR-based tests, the current gold standard for COVID-19 diagnosis. A report describing the development and validation of the test appears in Science Advances.
“This test addresses the critical needs for a rapid, ultrasensitive COVID-19 diagnosis along with effective large-scale screening efforts,” says corresponding author Tony Hu, chair in biotechnology innovation at Tulane University School of Medicine.
“Our development can quickly identify patients who have the virus, which is required to help address the ongoing threat to public health worldwide.”
The assay platform can detect very small amounts of SARS-CoV-2 virus RNA in saliva by leveraging CRISPR, the revolutionary gene editing technology, to amplify assay signal. The technology doesn’t require an RNA isolation step used in PCR tests.
The new test involves mixing saliva with an assay solution on an assay chip and heating it to amplify a small region of viral RNA. A modified CRISPR complex that contains a “guide” RNA specific for this virus RNA region rapidly binds and cuts both this amplified RNA region and a tagged DNA probe to produce a fluorescent signal that the smartphone device reads.
The assay is faster, more sensitive, and more user-friendly than standard PCR tests, while requiring fewer steps and less equipment. A prototype smartphone-based fluorescent microscope device designed for point-of-care use reads the assay.
“The sensitivity and simplicity of this test, its straightforward sample collection procedure, and the inexpensive nature of the readout device should permit the rapid translation of this approach to COVID-19 testing efforts once we obtain FDA approval,” Hu says.
Source: Tulane University