The world’s most detailed atlas of the genetic code of the human retina could help treat and prevent blindness, researchers report.
Here, Raymond Wong, unit head of the cellular reprogramming unit at the Centre for Eye Research Australia and principal investigator in ophthalmology at the University of Melbourne, explains the research:
Many different things can cause blindness.
Often, it’s related to aging with conditions like glaucoma, age-related macular degeneration or severe cataracts stealing sight. Sometimes it’s the result of eye injury or infection, or a complication of diabetes.
But for younger people, the cause of blindness is most often genetic. Millions of working-age people worldwide are affected by inherited retinal diseases (IRDs) like retinitis pigmentosa, macular dystrophy, or a range of rarer genetic conditions.
The University of Melbourne and Centre for Eye Research Australia have contributed to a new genetic map of the retina, revealing vital clues that will aid future research to prevent and treat blindness.
The map is the world’s most detailed look at the genetic code of the human retina.
Researchers worldwide will be able to use this genetic atlas to gain unprecedented insights into eye disease, opening up new approaches to preventing and treating blindness.
The retina is the thin layer of tissue at the back of the eye, made up of millions of cells that work together to process light and transmit signals to the brain via the optic nerve. Vision loss from inherited retinal diseases can be sudden or gradual, and partial or complete and there is a lot we still don’t know about what causes these diseases. This makes preventing and treating blindness a significant scientific challenge.
What is inherited retinal disease?
To understand blindness, let’s first look at how vision works. Essentially, the eye functions like a camera, capturing light and converting it to electrical signals for the brain to interpret as images.
This all happens in a tiny fraction of a second—so fast we comprehend it as instant. But, if any part of this complex process is impaired, vision loss can occur.
For inherited retinal diseases, the problem occurs in the retina. Inherited retinal diseases occur when genetic “mistakes” cause retinal cells to stop functioning correctly. Over time, the photoreceptor (light-sensing) cells can die, leading to vision loss and blindness.
More than 200 genes are known to be associated with retinal diseases. These genetic errors can be passed down from parent to child, but this doesn’t always result in disease. This means an inherited retinal disease can strike even when there is no known family history of it. It seems to come out of nowhere but the genetic imperfection has been silently lurking in the family tree.
Making a retina atlas
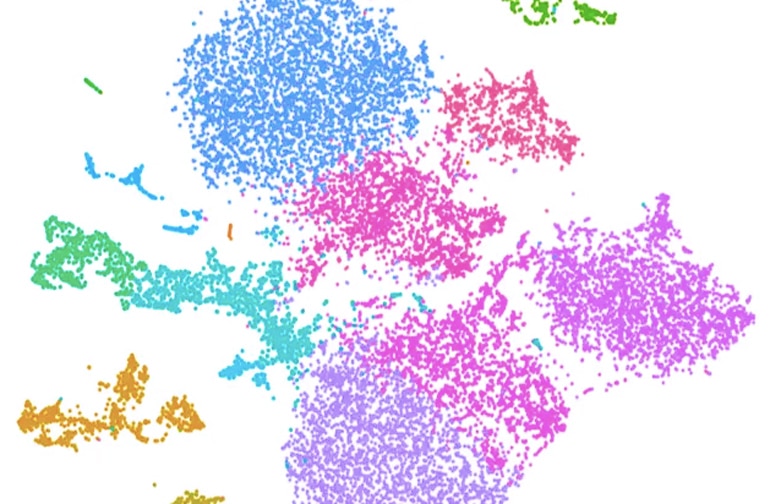
Our new retinal atlas provides new insights into the genetic code of the cells in the human retina.
Our research, published this week in European Molecular Biological Organisation Journal, is a collaboration which I led with Samuel Lukowski from the Institute for Molecular Science at the University of Queensland and Joseph Powell, associate professor from the Garvan Institute of Medical Research.
To create this map, we examined the complex genetic sequences behind more than 20,000 individual retinal cells. This allowed us to develop a genetic profile of the major cell types in the retina, and the genes they express to function normally.
Cells mapped include photoreceptors, which sense light and allow people to see, as well as retinal ganglion cells, which transmit messages to the brain along the optic nerve, and other cells that support the function and stability of the retina.
This study is part of the Human Cell Atlas, a global project that aims to create comprehensive reference maps of all human cells to better understand, diagnose, and treat disease. The retina is the first part of the eye to be mapped in the project.
Baseline for comparison
Now that we have a detailed map of a healthy retina, we can use this to advance our understanding of what goes wrong in inherited retinal diseases.
Having a detailed gene profile of individual retinal cell types will help us study how these genes impact different kinds of cells, and what genetic signals cause a cell to stop functioning, leading to vision loss and blindness.
The atlas will also help scientists exploring the emerging area of cell therapy, which could replace faulty retinal cells with new cells developed from stem cells in the lab.
The retinal cell map will give scientists a clear benchmark to assess the quality of cells derived from stem cells and to determine whether they have the correct genetic code to enable them to function. With this new knowledge, we’re one step closer to better identifying what causes blinding eye disease, and to ultimately developing treatments and cures.
Source: University of Melbourne



