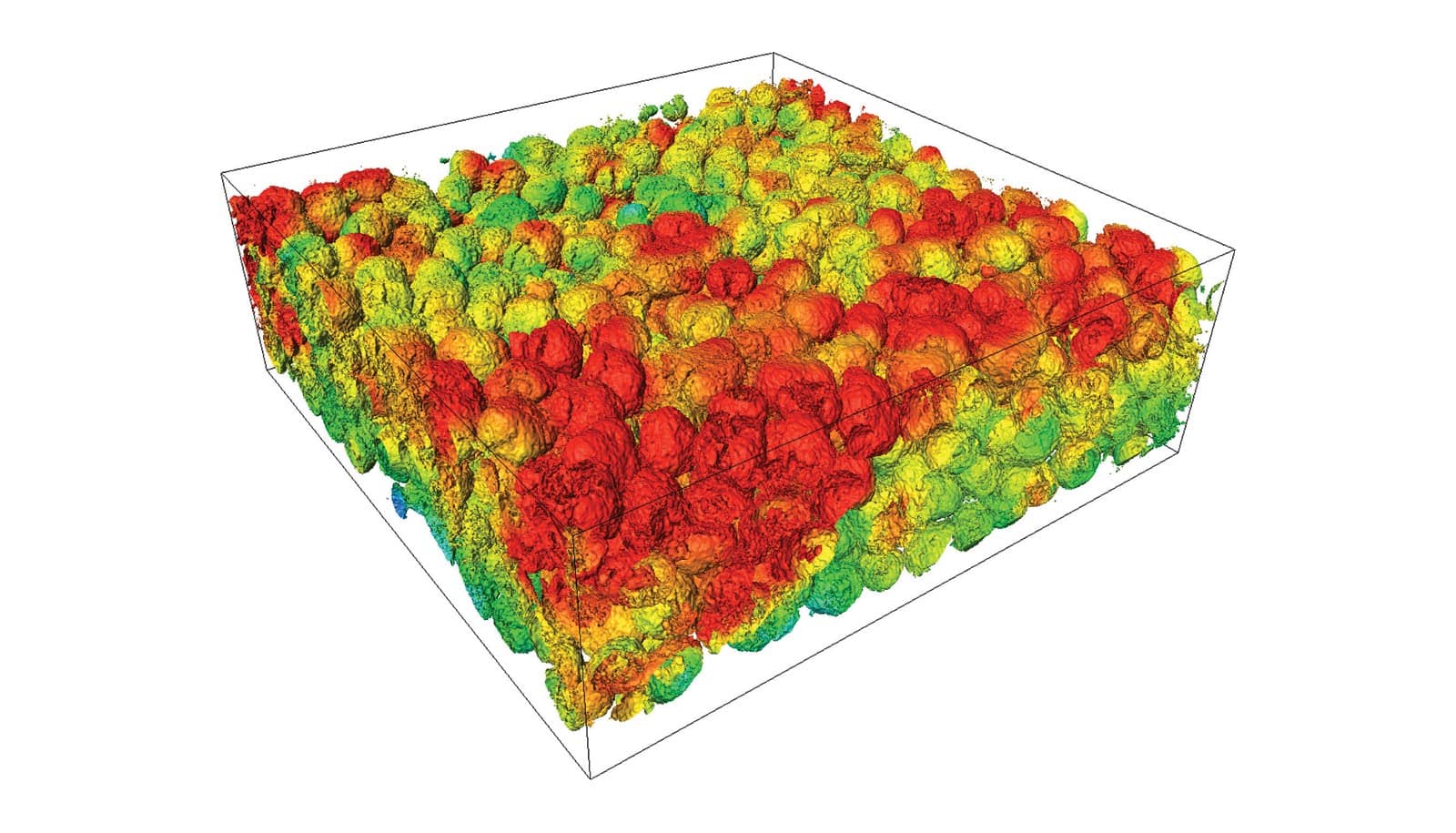
The most comprehensive view yet of lithium-ion battery electrodes could lead to more reliable, long-lasting power for your smartphone or car, say researchers.
The technology has been extremely slow to improve. While electric cars can more than handle the average American’s daily commute, the average gas-powered car can still go farther on a full tank of gas, charging stations are scarce, and it takes significantly longer to charge a battery than to fill a tank.
To improve charging capacity in lithium-ion batteries and increase adoption of electric cars, the industry will have to return to the basic science of how batteries wear out over time.
“The creation of knowledge is sometimes more valuable than solving the problem of battery electrode damage,” says Kejie Zhao, an assistant professor of mechanical engineering at Purdue University. “Before, people didn’t have the techniques or theory to understand this problem.”

AI and X-ray
The technique, explained in the journals Advanced Energy Materials and the Journal of the Mechanics and Physics of Solids, is essentially an artificial intelligence-driven X-ray tool. It can automatically scan thousands of particles in a lithium-ion battery electrode at once—all the way down to the atoms that make up the particles themselves—using machine-learning algorithms.
Granted, there are actually millions of particles in a battery electrode. But researchers can now analyze them more thoroughly than they could before—and at the various operating conditions that we use commercial batteries in the real world, such as their voltage window and how quickly they charge.
“Most work had been focused on the single particle level and using that analysis to understand the whole battery. But there’s obviously a gap there; a lot differs between a single particle at a micron scale and the whole battery at a much larger scale,” says Zhao, whose lab studies the fundamental science of how the mechanical and electrochemical aspects of a battery affect each other.
Inside a charging battery
Every time that a battery charges, lithium ions travel back and forth between a positive electrode and a negative electrode. These ions interact with particles in electrodes, causing them to crack and degrade over time. Electrode damage reduces a battery’s charging capacity.
It’s hard for a battery to have a high capacity and be reliable at the same time, Zhao says. Increasing a battery’s capacity often means sacrificing its reliability.
The researchers’ work to map out damage in lithium-ion batteries started with their finding that degradation in battery particles doesn’t happen at the same time or in the same location; some particles fail more quickly than others.
But to truly study this in more detail, the team needed to create a new technique altogether; existing methods wouldn’t entirely capture damage in battery electrodes.
The researchers turned to massive, miles-long facilities called synchrotrons at the European Synchrotron Radiation Facility (ESRF) and the Stanford Synchrotron Radiation Lightsource (SSRL) of SLAC National Laboratory. These facilities host particles traveling at almost the speed of light, giving off radiation that is used to create images called synchrotron X-rays.
Virginia Tech researchers manufactured the materials and batteries for testing—ranging from the pouch cell batteries in smartphones to the coin cells in watches. Researchers at ESRF and SSRL created the ability to scan as many electrode particles in these batteries as possible in a single go, then produce these X-ray images for analysis. Maps of particle cracking and degradation at the surfaces of particles, called “interfacial debonding,” can now serve as a reference tools for knowing ranging degrees of damage in battery electrodes.
To understand how these cracks impact battery performance, Zhao’s team at Purdue developed theories and computational tools. They found, for example, that because particles near where lithium ions shuttle back and forth, called the “separator,” are more used than particles near the bottom of electrode materials, they fail more quickly.
This variability in electrode particle damage, or “heterogeneous degradation,” is more severe in thicker electrodes and during fast-charging conditions.
“The capacity of batteries doesn’t depend on how many particles are in the battery; what matters is how the lithium ions are used,” Zhao says.
The goal for the project is not for every researcher and industry player to use the technique itself—especially given that there are only a handful of synchrotrons in the US—but for these groups to use the knowledge generated from the technique. The researchers plan to continue using the technique to document how damage happens and affects performance in commercial batteries.
Support for the work came from the US Department of Energy and the National Science Foundation among others.
Source: Purdue University