Scientists are counting on carbon nanotube films to make lithium batteries charge faster and last longer.
New research shows the thin nanotube films effectively stop dendrites that grow naturally from unprotected lithium metal anodes in batteries. Over time, the tentacle-like dendrites can pierce the battery’s electrolyte core and reach the cathode, causing the battery to fail.
That problem has both dampened the use of lithium metal in commercial applications and encouraged researchers worldwide to solve it.
Lithium metal charges much faster and holds about 10 times more energy by volume than the lithium-ion electrodes found in just about every electronic device, including cellphones and electric cars.
“One of the ways to slow dendrites in lithium-ion batteries is to limit how fast they charge,” says James Tour, chemistry chair and a professor of computer science and of materials science and nanoengineering at Rice University. “People don’t like that. They want to be able to charge their batteries quickly.”
The answer, which researchers detail in Advanced Materials, is simple, inexpensive, and highly effective at stopping dendrite growth, Tour says.
“What we’ve done turns out to be really easy,” he says. “You just coat a lithium metal foil with a multiwalled carbon nanotube film. The lithium dopes the nanotube film, which turns from black to red, and the film in turn diffuses the lithium ions.”
“Physical contact with lithium metal reduces the nanotube film, but balances it by adding lithium ions,” says postdoctoral researcher Rodrigo Salvatierra, co-lead author with graduate student Gladys López-Silva. “The ions distribute themselves throughout the nanotube film.”
When the battery is in use, the film discharges stored ions and the underlying lithium anode refills it, maintaining the film’s ability to stop dendrite growth.
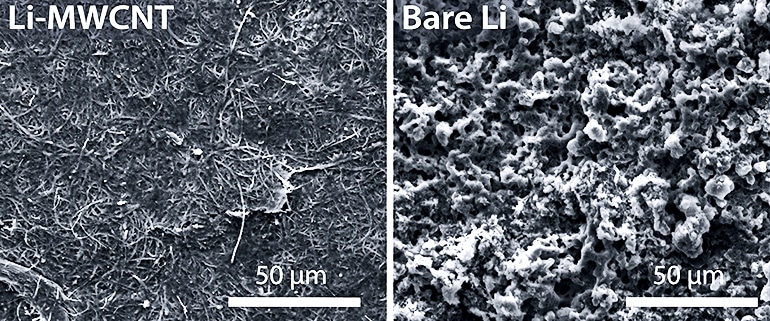
The tangled-nanotube film effectively quenched dendrites over 580 charge/discharge cycles of a test battery with a sulfurized-carbon cathode the lab developed in previous experiments. The full lithium metal cells retained 99.8 percent of their coulombic efficiency, the measure of how well electrons move within an electrochemical system.
Additional coauthors are from Rice; the King Fahd University of Petroleum and Minerals, Saudi Arabia; the Korea Basic Science Institute; and the McGovern Medical School at the University of Texas Health Science Center.
The Air Force Office of Scientific Research; the National Institutes of Health; the National Council of Science and Technology, Mexico; the National Council for Scientific and Technological Development; Ministry of Science, Technology and Innovation and Coordination for the Improvement of Higher Education Personnel, Brazil; and Celgard, LLC funded the work.
Source: Rice University



