Researchers have delineated the architecture of the nuclear pore complex in yeast cells.
The biological blueprint they uncovered shares principles sometimes seen on a much larger scale in concrete, steel, and wire.
“It reminds us of a suspension bridge, in which a combination of sturdy and flexible parts produce a stress-resilient structure,” says Michael P. Rout, professor at Rockefeller University.
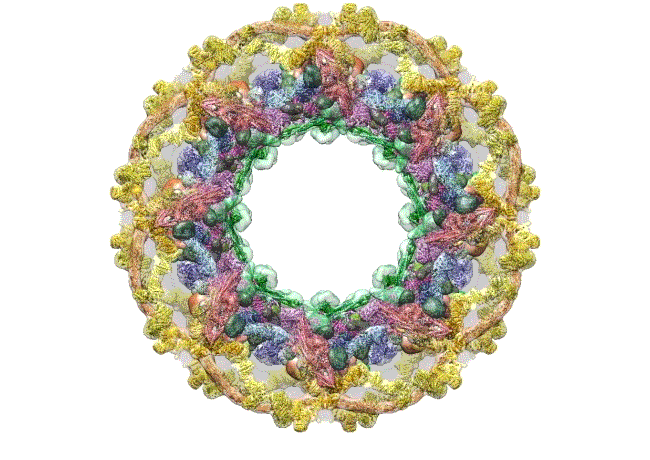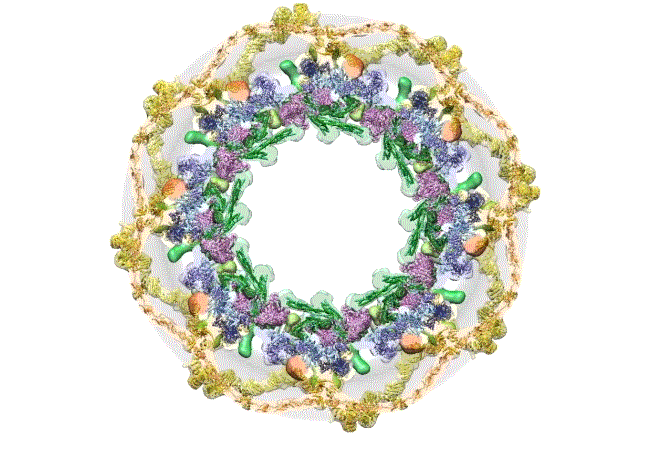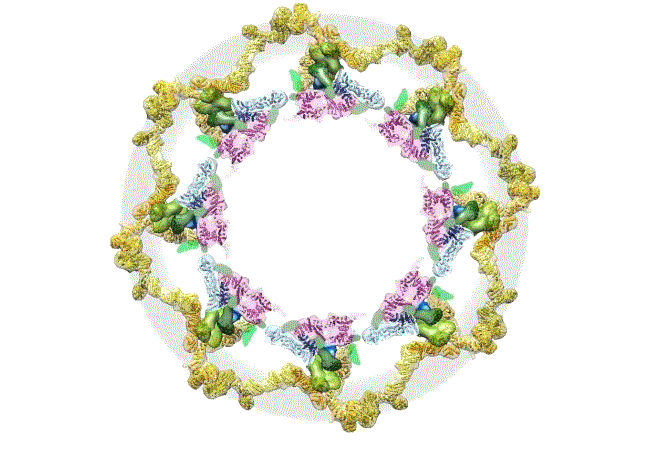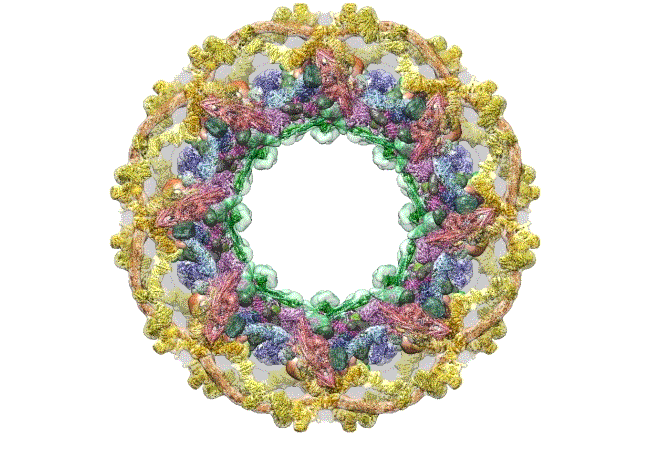
The pore complex contains 552 component proteins, called nucleoporins. Scientists hadn’t previously known how they all fit together. It took a combination of approaches to assemble a comprehensive map of these pieces. The researchers hope the molecular structure will lead to new studies of how the nuclear portal functions normally, and how defects in it lead to diseases such as cancer.
An ancient structure
The pore complex first emerged when single-celled organisms—the only living things at the time—acquired special compartments containing organ-like structures, including the nucleus, which houses the cell’s genetic code.
It serves not only as a conduit to and from the nucleus, but also as a checkpoint regulating what passes in and out. Genetic instructions transcribed into RNA can exit, for example, while proteins needed inside the nucleus may enter. Other things, such as viruses bent on taking over the cell, are kept at bay.
The researchers began mapping this ancient structure more than 20 years ago, knowing the project could well span decades since the target of their curiosity is not easily defined.
More than a third of the pore complex can move about, and this flexibility, along with the structure’s immense size and the constant stream of traffic passing through it, meant that no single approach to mapping it would work.
“In the end, we used everything we could lay our hands on, brought the results together, and integrated them into a single structure,” says study co-leader and professor Brian T. Chait.
The team determined the type and amount of each nucleoporin and their proximities to one another, as well as the weight and shape of the whole complex.
How enzymes direct ‘traffic’ inside cells
This data allowed them to visualize the anatomy of many of the individual pore components and to place them all within the pore complex. They uncovered a complicated ringed structure containing rigid, diagonal columns and flexible connectors that evoke the towers and cables of human-made structures like the Golden Gate Bridge.
The resulting map is a breakthrough in a line of investigation with a deep history. The pore complex first came into human view in the 1950s, when Rockefeller scientist Michael Watson observed small densities dotting the surface of the nuclear envelope. And about two decades later, the lab of Günter Blobel, who passed away last month, was among the first to discover individual nups and then determine their structure.
Why it matters
When it comes to the pore complex, yeast has a considerable amount in common with humans. When the team compared their data with structural findings from human pore complexes, they found similar elements arranged somewhat differently. The resemblance suggests the yeast pore complex could be useful for research relevant to humans.
And there’s a lot of such research to be done. Defects in the pore complex and its components have been linked to a host of diseases, including autoimmune disorders and cancer; meanwhile, viruses have evolved ways to sneak past it altogether. But the details of these malfunctions and blind spots are often obscure.
Scientists control tiny ‘factories’ in engineered cells
The new yeast structure may help. With it, the team found they could map sites that are altered in some cancers—evidence, they say, that the yeast pore complex can be used to test how factors like stress, drugs, or mutations change the human structure, and so aiding efforts to understand and treat disease.
Researchers from University of California, San Francisco; Boston University Medical School; and Baylor College of Medicine also contributed to the research.
Source: Rockefeller University



