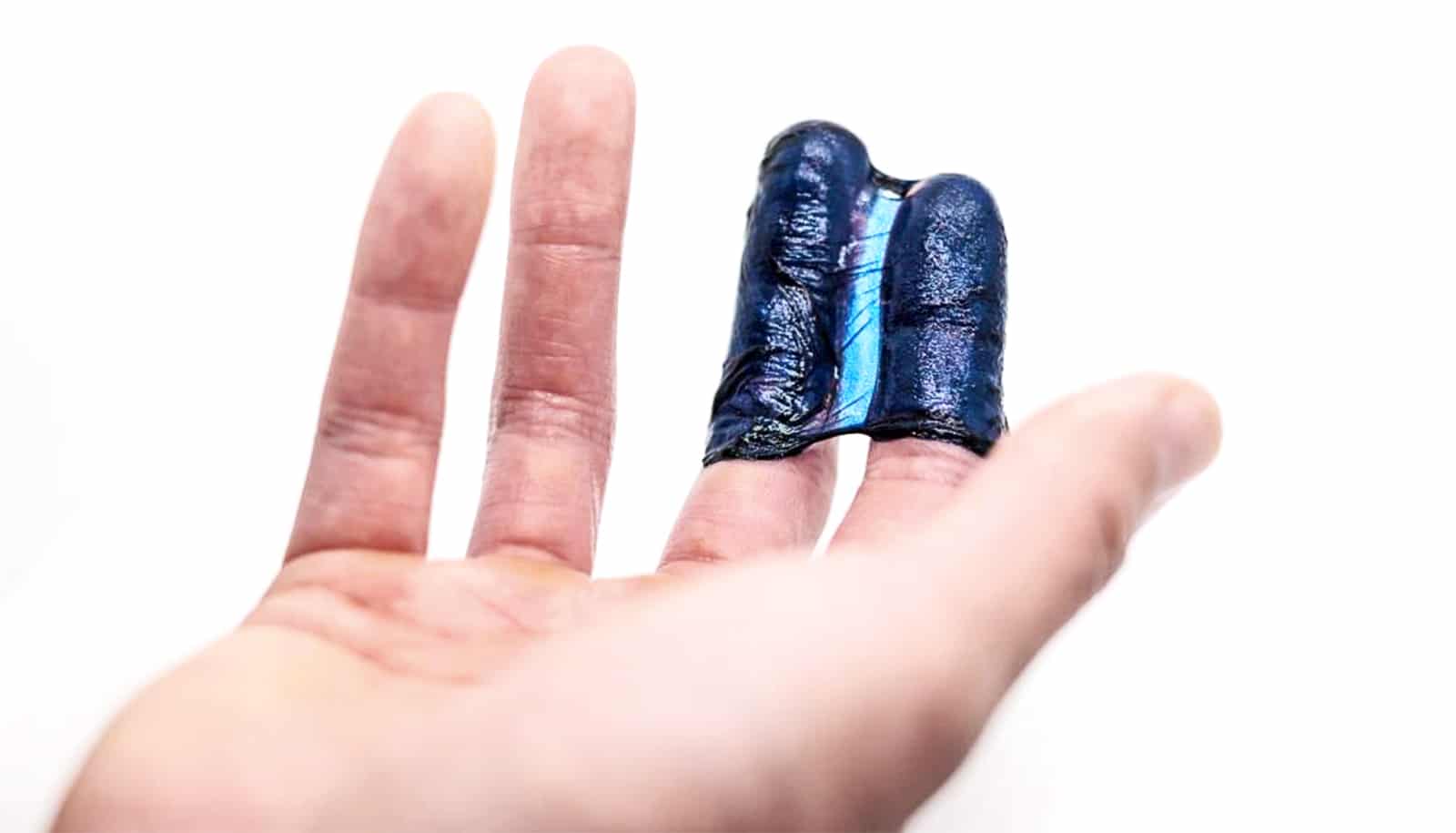
Researchers have created an injectable bandage to stop bleeding and promote wound healing.
A penetrating injury from shrapnel is a serious obstacle in overcoming battlefield wounds that can ultimately lead to death. Given the high mortality rates due to hemorrhaging, there is an unmet need to quickly self-administer materials that prevent fatality due to excessive blood loss.
The new bandage uses kappa-carrageenan and nanosilicates to form injectable hydrogels to promote hemostasis (the process to stop bleeding) and facilitate wound healing via a controlled release of therapeutics. The researchers created the injectable bandage with a gelling agent commonly used in preparing pastries.
“Injectable hydrogels are promising materials for achieving hemostasis in case of internal injuries and bleeding, as these biomaterials can be introduced into a wound site using minimally invasive approaches,” says Akhilesh K. Gaharwar, assistant professor in the biomedical engineering department at Texas A&M University.
“An ideal injectable bandage should solidify after injection in the wound area and promote a natural clotting cascade. In addition, the injectable bandage should initiate wound healing response after achieving hemostasis,” he says.
The study uses a commonly used thickening agent known as kappa-carrageenan, obtained from seaweed, to design injectable hydrogels. Hydrogels are a 3D water swollen polymer network, similar to Jell-O, simulating the structure of human tissues.
When kappa-carrageenan is mixed with clay-based nanoparticles, injectable gelatin is the result. The charged characteristics of clay-based nanoparticles provide hemostatic ability to the hydrogels. Specifically, plasma protein and platelets form blood adsorption on the gel surface and trigger a blood clotting cascade.
Drug delivery gel can heal without the drugs
“Interestingly, we also found that these injectable bandages can show a prolonged release of therapeutics that can be used to heal the wound” says Giriraj Lokhande, a graduate student in Gaharwar’s lab and first author of the paper. “The negative surface charge of nanoparticles enabled electrostatic interactions with therapeutics thus resulting in the slow release of therapeutics.”
The results appear in Acta Biomaterialia.
The National Science Foundation’s Chemical, Bioengineering, Environmental, and Transport Systems Division and the National Institutes of Health’s National Institute of Biomedical Imaging and Bioengineering funded the research.
Source: Marcus Misztal for Texas A&M University