Scientists have identified a possible new route to regenerating insulin-producing beta cells in the pancreas. The findings offer insight into the basic mechanisms behind healthy metabolism and diabetes.
In people with type I diabetes, beta cells in the pancreas die and don’t return. Without these cells, the body loses the ability to control blood glucose.
“We’ve seen phenomenal advances in the management of diabetes, but we cannot cure it,” says Mark Huising, assistant professor of neurobiology, physiology, and behavior in the University of California, Davis College of Biological Sciences. “If you want to cure the disease, you have to understand how it works in the normal situation.”
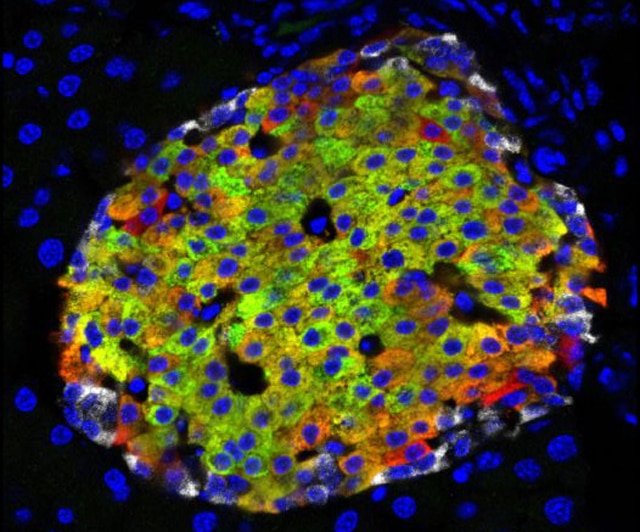
Working with both laboratory mice and human tissue, Huising is studying how the cells in the islets of Langerhans in the pancreas work together to regulate blood glucose. In both mice and people, the islets contain beta cells, which detect glucose and secrete insulin, and other cell types including alpha cells that produce glucagon, a hormone that raises blood sugar. The opposite effects of insulin and glucagon enable the body to regulate blood sugars and store nutrients.
Type I diabetes is a disease with two parts. Firstly, the body’s own immune system kills beta cells, and then they fail to regenerate (or those that do are killed). An effective cure for type I diabetes would involve dealing with both problems.
Accepted dogma, Huising says, has been that new beta cells are generated by other beta cells dividing. But now by applying new techniques in microscopy, his team has discovered, scattered around the edges of the islets, another type of cell that looks a lot like an immature beta cell.
How a person’s own fat could one day cure diabetes
These new cells can make insulin, but don’t have the receptors to detect glucose, so they can’t function as a full beta cell. However, Huising’s team was able to observe alpha cells in the islet turn into immature beta cells and then mature into real beta cells.
“There’s much more plasticity in the system than was thought,” says Huising, senior author of a paper on the work in the journal Cell Metabolism.
It’s an exciting result for three reasons, Huising says. Firstly, this is a new beta cell population in both humans and mice that wasn’t known before. Secondly, the new population could be a source to replenish beta cells killed off in diabetes. Finally, understanding how these cells mature into functioning beta cells could help in developing stem cell therapies for diabetes. Stem cells have the potential to develop into a wide range of other cells. So far, attempts to grow real beta cells from stem cells have made great strides, but these efforts have not yet reached their full potential because they get hung up at an earlier immature stage.
This basic understanding of cells in the islets could also help in understanding type II diabetes, where beta cells do not die but become inactive and no longer secrete/release insulin.
“The concept of harnessing the plasticity in the islet to regenerate beta cells has emerged as an intriguing possibility in recent years,” says Andrew Rakeman, director of discovery research at the Juvenile Diabetes Research Foundation, which supported the research. “The work from Dr. Huising and his team is showing us not only the degree of plasticity in islet cells, but the paths these cells take when changing identity.
“Adding to that the observations that the same processes appear to be occurring in human islets raises the possibility that these mechanistic insights may be able to be turned into therapeutic approaches for treating diabetes.”
Coauthors of the paper are from UC Davis; the Salk Institute for Biological Studies, La Jolla, California; Amanda Ackermann, The Children’s Hospital of Philadelphia; and the University of Pennsylvania. The Hartwell Foundation for Biomedical Research also funded the work.
Source: UC Davis



