Researchers have developed a type of wearable device they call a “biosymbiotic device,” which has several unprecedented benefits.
Wearable sensors to monitor everything from step count to heart rate are nearly ubiquitous. But for scenarios such as measuring the onset of frailty in older adults, promptly diagnosing deadly diseases, testing the efficacy of new drugs, or tracking the performance of professional athletes, medical-grade devices are needed.
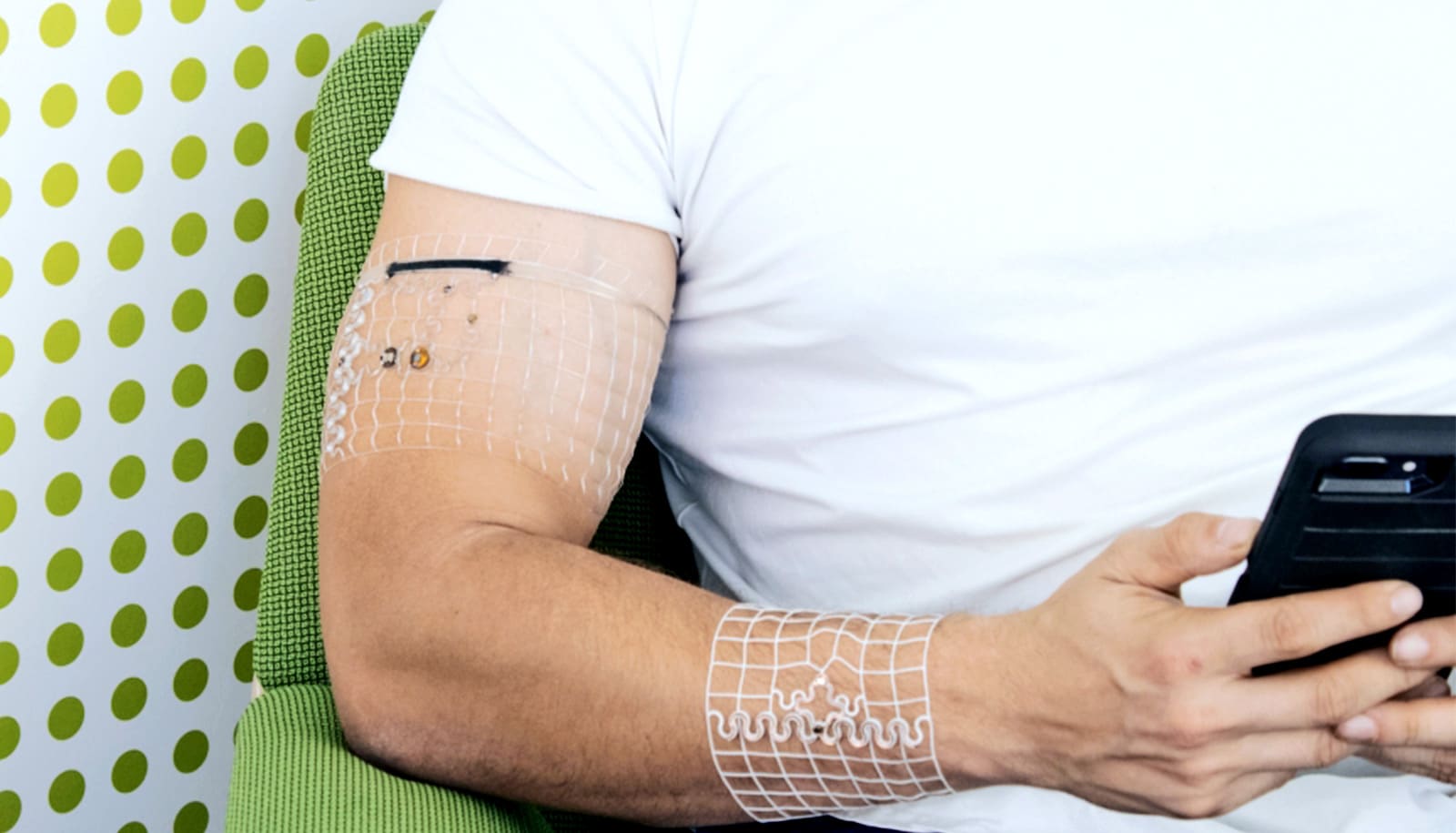
Not only are the new devices custom 3D-printed and based on body scans of wearers, but they can operate continuously using a combination of wireless power transfer and compact energy storage.
“It’s as simple as putting the device on. Then you forget about it, and it does its job.”
“There’s nothing like this out there,” says Philipp Gutruf, assistant professor of biomedical engineering, faculty fellow in the College of Engineering, and a member of the BIO5 Institute at the University of Arizona. “We introduce a completely new concept of tailoring a device directly to a person and using wireless power casting to allow the device to operate 24/7 without ever needing to recharge.”
Current wearable sensors face various limitations. Smartwatches, for example, need to be charged, and they can only gather limited amounts of data due to their placement on the wrist. By using 3D scans of a wearer’s body, which can be gathered via methods including MRIs, CT scans, and even carefully combined smartphone images, Gutruf and his team can 3D-print custom-fitted devices that wrap around various body parts.
Imagine a virtually unnoticeable, lightweight, breathable, mesh cuff designed specifically for your bicep, calf, or torso. The ability to specialize sensor placement allows researchers to measure physiological parameters they otherwise couldn’t.
“If you want something close to core body temperature continuously, for example, you’d want to place the sensor in the armpit. Or, if you want to measure the way your bicep deforms during exercise, we can place a sensor in the devices that can accomplish that,” says Tucker Stuart, a doctoral student in biomedical engineering and first author on the paper. “Because of the way we fabricate the device and attach it to the body, we’re able to use it to gather data a traditional, wrist-mounted wearable device wouldn’t be able to collect.”
Because these biosymbiotic devices are custom fitted to the wearer, they’re also highly sensitive. Gutruf’s team tested the device’s ability to monitor parameters including temperature and strain while a person jumped, walked on a treadmill, and used a rowing machine. In the rowing machine test, subjects wore multiple devices, tracking exercise intensity and the way muscles deformed with fine detail. The devices were accurate enough to detect body temperature changes induced by walking up a single flight of stairs.
Gutruf and his team aren’t the first to adapt wearables to track health and body function. However, current wearable devices do not have the ability to track metrics continuously, or with enough precision to make medically meaningful conclusions.
Some wearables used by researchers are patches that stick to the skin, but they come off when skin goes through its normal shedding process, or sometimes when a subject sweats. Even highly sophisticated wearable devices used in clinical settings, such as ECG monitors, face these issues. Also, they aren’t wireless, which severely limits mobility. Patients can’t go about their normal daily routines if they’re tethered to bulky external devices.
The biosymbiotic device that Gutruf’s team has introduced uses no adhesive, and it receives its power from a wireless system with a range of several meters. The device also includes a small energy storage unit, so that it will function even if the wearer goes out of the system’s range, including out of the house.
“These devices are designed to require no interaction with the wearer,” Gutruf says. “It’s as simple as putting the device on. Then you forget about it, and it does its job.”
The research appears in the journal Science Advances.
Funding for the research came from the Flinn Foundation Translational Bioscience Seed Grants Pilot Program. The team has also been working with Tech Launch Arizona, the commercialization arm of the university, to protect the intellectual property and launch a startup to bring the technology to market.
Source: University of Arizona