A new preoperative test for thyroid cancer is faster and about two-thirds more accurate than the diagnostic tests doctors use today, report researchers.
Although more validation will be necessary before doctors can use it clinically, the new metabolic thyroid test shows promise for preventing thousands of unnecessary thyroid removals each year, such as the partial removal Amanda Helms had due to an inconclusive test.
“All the uncertainty was nerve wracking,” says Helms, a grad student in chemistry at the University of Texas at Austin.
“If we could prevent people from having surgery they don’t need and enable them to have a more precise diagnosis, we can improve treatment for patients and lower costs for the health care system,” says Livia S. Eberlin, assistant professor of chemistry and diagnostic medicine at UT Austin and co-principal investigator.
Rachel DeHoog, a graduate student who worked on the study, adds, “Also very importantly, the ability to have certainty in your diagnosis is transformative for a patient presented with the grueling possibility of having cancer.”
Tackling uncertainty
Each year in the US, about 52,000 people receive a diagnosis of thyroid cancer. Unfortunately, the test used for diagnosis, called fine needle aspiration (FNA), is inconclusive about 1 out of every 5 times. When a pathologist can’t confirm the presence of cancer, the patient may receive a follow-up genetic test that itself can produce false positive results.
Given the uncertainties, doctors often recommend removing part or all of the thyroid—the gland in the neck producing hormones that control the body’s metabolic rate, as well as heart and digestive function, muscle control, brain development, mood, and bone maintenance. Thousands of patients each year have the surgery only to later learn it was unnecessary.
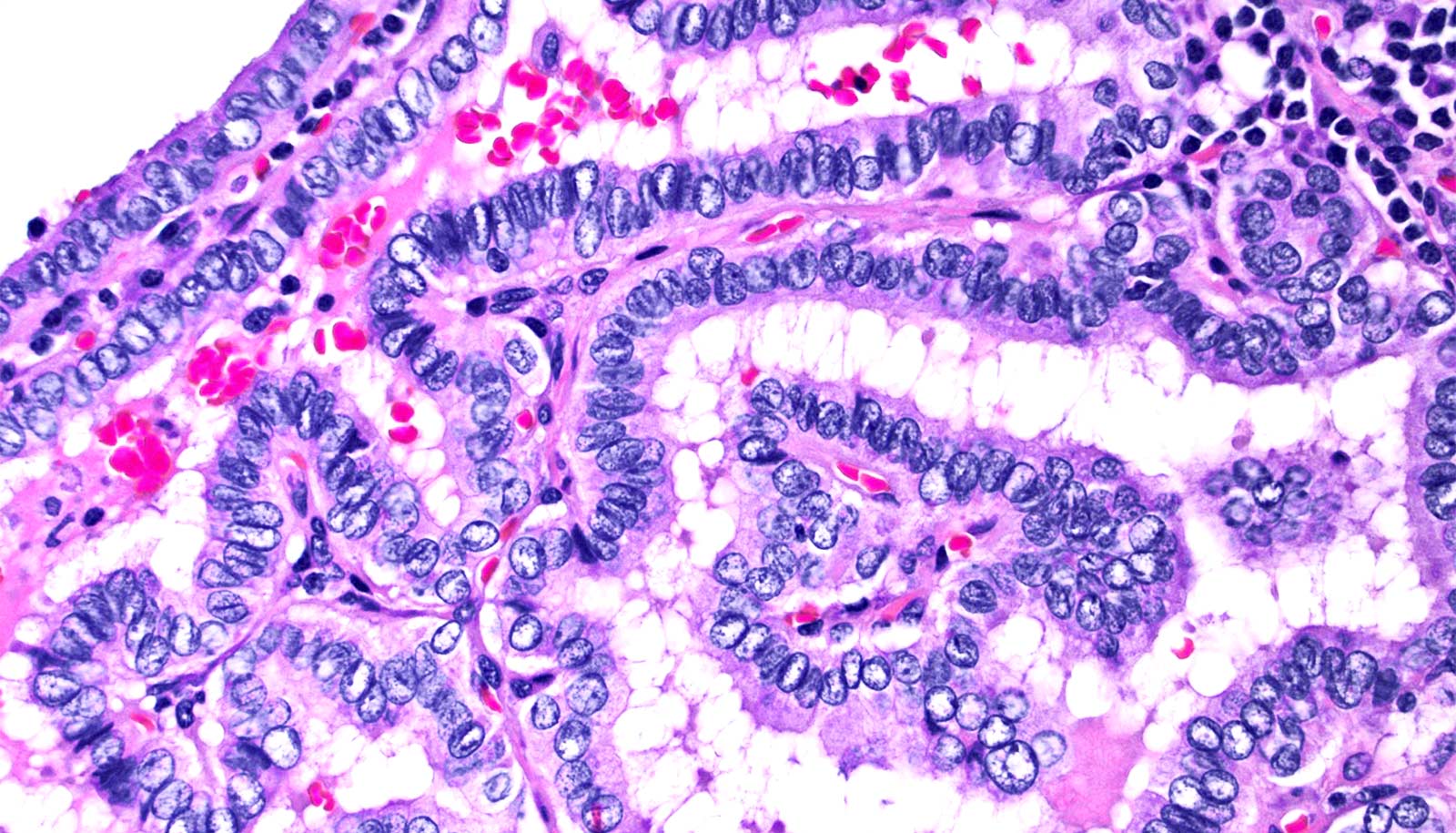
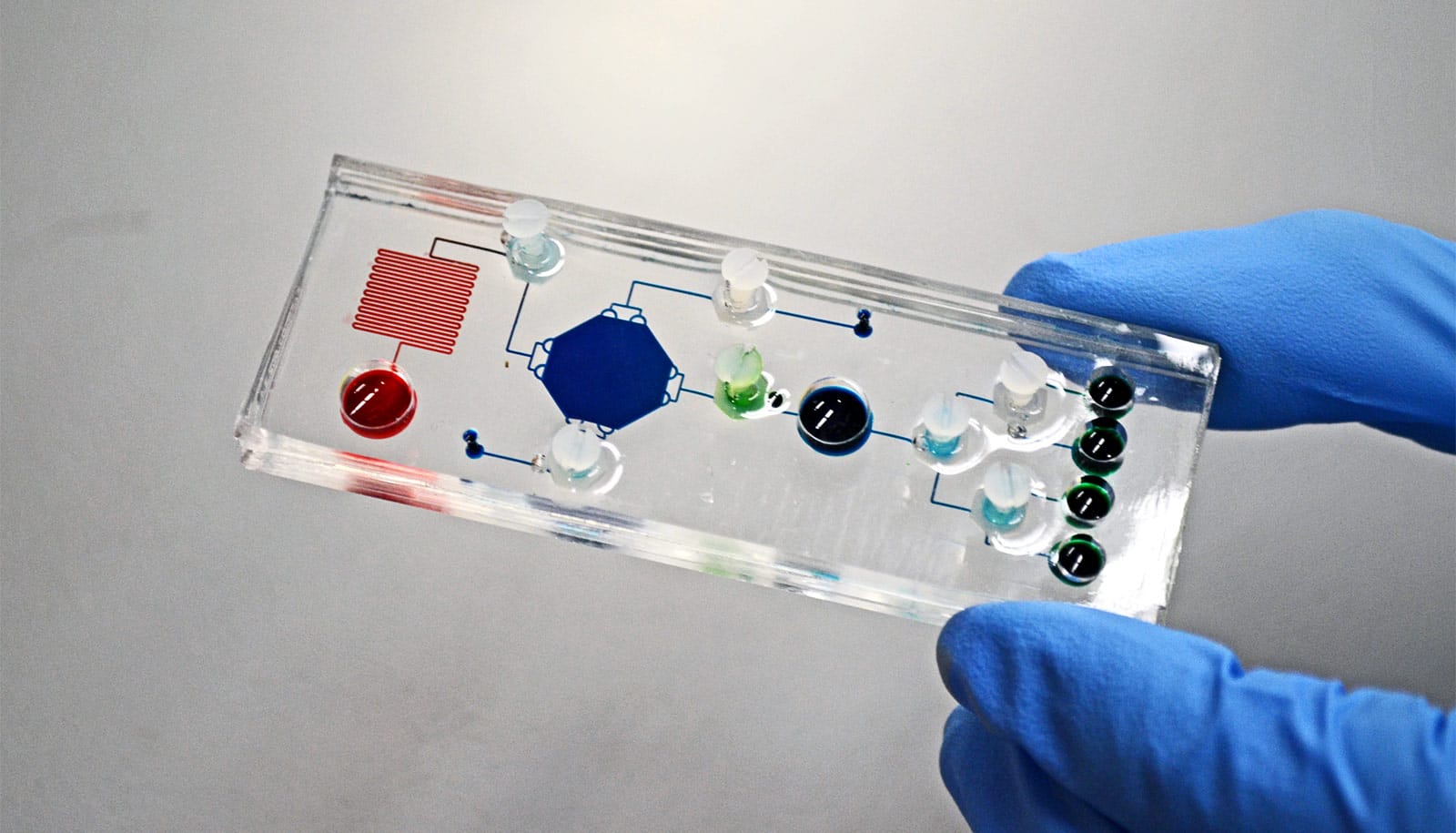
Using a technology called mass spectrometry imaging, the new metabolic thyroid test identifies metabolites cancerous cells produce that act as a kind of diagnostic fingerprint. The researchers worked on identifying these diagnostic metabolic fingerprints for over two years using 178 patient tissues before starting a pilot clinical study.
During the clinical study, they tested 68 new patients, nearly a third of whom had received inconclusive FNA results. The new metabolic thyroid test returned a false positive only about 1 time in 10 and could have prevented 17 patients in the study from undergoing unnecessary surgeries.
Avoiding unnecessary surgery
The improved accuracy would prevent unnecessary surgeries, many of which lead patients to need hormone replacement therapy for the rest of their lives or to have to cope with other consequences of having all or part of their thyroid removed.
For example, Helms learned a few years ago as an undergraduate student at the age of 19 that lumps on her thyroid were indicative of possible cancer. When her FNA biopsy came back as inconclusive, a doctor recommended removing the right half of her thyroid. For Helms, a difficult surgery and recovery followed, though she soon received word that pathologists detected no cancer in the removed tissue. Helms says the new test could prevent others from going through what she did.
“A lot fewer people would have to get surgery unnecessarily, which saves them time, money, and recovery,” Helms says. “It would also take away the uncertainty.”
James Suliburk, a coprincipal investigator and head of endocrine surgery at Baylor College of Medicine in Houston, collected FNA biopsies from the patients involved in the study and implemented the technology in a pilot trial to demonstrate the accuracy of the new test.
“With this next generation test, we can provide thyroid cancer diagnoses faster and with more precision than current techniques—this will be the new state-of-the-art,” says Suliburk, who operates at Baylor St. Luke’s Medical Center and Harris Health System’s Ben Taub Hospital. “We are able to do this analysis directly on the FNA sample and much more rapidly than the current process, which could take between three and 30 days.”
The team is now preparing to start a two-year validation study on FNAs from about a thousand new patients collected in the US, Brazil, and Australia. If the results hold up, they hope the technology will be translated to the clinic as a routine diagnostic tool.
The results appear in the journal Proceedings of the National Academy of Sciences. Additional coauthors are from UT Austin, Baylor College of Medicine, the University of Sydney in Australia, and Stanford University.
Coauthors Eberlin, Suliburk, Wendong Yu, Jialing Zhang, Rachel DeHoog, and Elizabeth Alore are inventors on a provisional patent application owned by the Board of Regents of the University of Texas System and Baylor College of Medicine that relates to the use of mass spectrometry to diagnose thyroid cancer.
Funding for the study came from the Cancer Prevention & Research Institute of Texas (CPRIT) through an Early Translation Research Award to Eberlin and Suliburk.
Source: University of Texas at Austin