Scientists have created a nanoscale light detector that can convert light to energy, combining both a unique fabrication method and light-trapping structures.
In today’s increasingly powerful electronics, tiny materials are a must as manufacturers seek to increase performance without adding bulk. Smaller is also better for optoelectronic devices—like camera sensors or solar cells—which collect light and convert it to electrical energy.
Think, for example, about reducing the size and weight of a series of solar panels, producing a higher-quality photo in low lighting conditions, or even transmitting data more quickly.
However, two major challenges have stood in the way: First, shrinking the size of conventionally used “amorphous” thin-film materials also reduces their quality. And second, when ultrathin materials become too thin, they are almost transparent—and actually lose some ability to gather or absorb light.
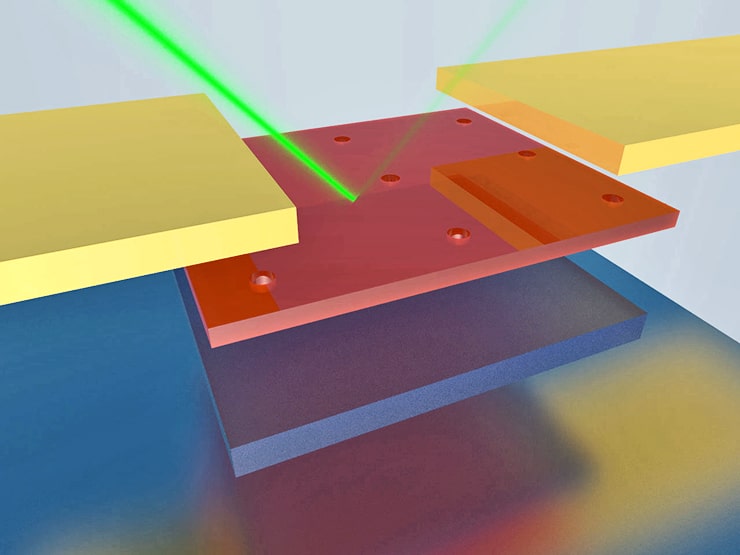
The new nanoscale light detector, a single-crystalline germanium nanomembrane photodetector on a nanocavity substrate, could overcome both of these obstacles.
“…you want to use a very thin material to realize the same function of devices in which you need to use a very thick material…”
“We’ve created an exceptionally small and extraordinarily powerful device that converts light into energy,” says Qiaoqiang Gan, associate professor of electrical engineering in the University at Buffalo’s School of Engineering and Applied Sciences and one of the paper’s lead authors. “The potential applications are exciting because it could be used to produce everything from more efficient solar panels to more powerful optical fibers.”
“The idea, basically, is you want to use a very thin material to realize the same function of devices in which you need to use a very thick material,” says Zhenqiang (Jack) Ma, professor in electrical and computer engineering at University of Wisconsin-Madison, also a lead author.
Nanocavities are made up of an orderly series of tiny, interconnected molecules that essentially reflect, or circulate, light.
The new device is an advancement of Gan’s work developing nanocavities that increase the amount of light that thin semiconducting materials like germanium can absorb. It consists of nanocavities sandwiched between a top layer of ultrathin single-crystal germanium and a bottom, reflecting layer of silver.
“Because of the nanocavities, the photons are ‘recycled’ so light absorption is substantially increased—even in very thin layers of material,” says Ma.
Microscope for dim light ‘recycles’ photons
However, most germanium thin films begin as germanium in its amorphous form—meaning that the material’s atomic arrangement lacks the regular, repeating order of a crystal. That also means that its quality isn’t sufficient for increasingly smaller optoelectronics applications.
An expert in semiconductor nanomembrane devices, Ma used a revolutionary membrane-transfer technology that allows him to easily integrate single crystalline semiconducting materials onto a substrate.
The result is a very thin, yet very effective light-absorbing photodetector—a building block for the future of optoelectronics.
“It is an enabling technology that allows you to look at a wide variety of optoelectronics that can go to even smaller footprints, smaller sizes,” says Zongfu Yu, who conducted its computational analysis for the project.
While the researchers demonstrated their advance using a germanium semiconductor, they can also apply their method to other semiconductors. “And importantly, by tuning the nanocavity, we can control what wavelength we actually absorb,” says Gan. “This will open the way to develop lots of different optoelectronic devices.”
Better light detector can see more colors
The researchers are applying jointly for a patent on the technology through the Wisconsin Alumni Research Foundation.
A paper describing the research appears in the journal Science Advances. Additional coauthors of the paper are from the University at Buffalo, the University of Wisconsin-Madison, and Yale University. The National Science Foundation partially supported this research.
Source: University at Buffalo



