Early findings from a new study could help in the development of immune-based treatments personalized to people with acute myeloid leukemia who are undergoing stem cell transplantation, researchers report.
“If you could identify and activate the immune cells from the stem cell donor that only target leukemia cells, and not normal, healthy cells, that would be a big win,” says Ben Vincent, an assistant professor in the School of Medicine division of hematology/oncology at the University of North Carolina-Chapel Hill and co-senior author of the paper, which appears in Blood Advances.
The new approach relies on the fact that a patient’s cells can have unique genetic signatures that produce proteins distinct from any proteins found in the stem cell donor. The patient’s proteins can serve as markers for the cancer cells’ destruction by the donor’s natural defense system, which is activated in the process of stem cell transplantation.
The researchers report that they were able to validate their method of using genetic sequencing and computer software to predict which of those patient sequences resulted in unique surface markers, or minor histocompatibility antigens. They confirmed the approach in a group of patients with myeloid leukemia.
The researchers tested whether their software could predict antigenic targets in a group of 101 leukemia patients who had undergone stem cell transplant. Using the software, they correctly identified 16 of 18 minor histocompatibility antigens that are known to occur in AML. In addition, they predicted more than 100 new minor histocompatibility antigen targets that could be expressed on an individual’s AML cells.
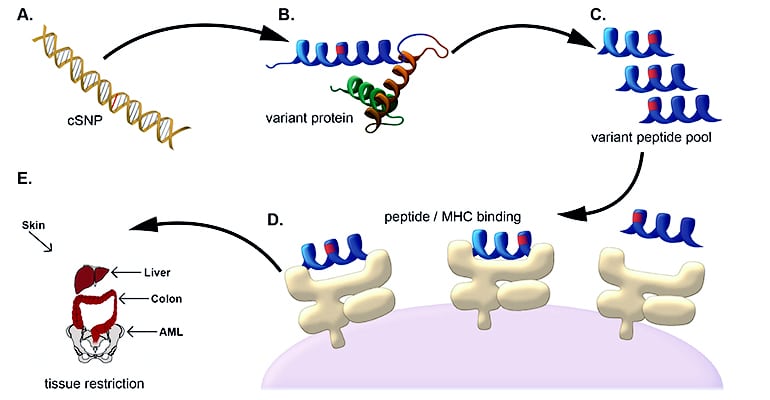
Because AML does not have one clear immune target, the validation of multiple potential targets is crucial. Based upon their computational predictions, the researchers confirmed a new minor histocompatibility antigen that is commonly presented on AML cells, and subsequently identified immune responses to this antigen in four of nine AML patients who had undergone stem cell transplant. Given these properties, the antigen could serve as a new target for immunotherapies across a wide range of AML patients.
Looking ahead, the researchers want to optimize their software to predict the most common AML-associated minor histocompatibility antigens present in the US population, and then confirm these predicted antigens as valid immunotherapy targets.
They could potentially use their predictions to engineer donor immune cells to specifically target the cancer cell antigens while preventing graft-versus-host disease, in which the donor’s immune cells attack healthy tissues.
“We’ve developed a software package that predicts leukemia-specific immune targets in any leukemia patient undergoing a stem cell transplant based on DNA and RNA sequencing and demonstrated that these data can lead to actual targets expressed on leukemia cells,” Vincent says.
“The next step of our work is to use that information for patient-specific therapies to try to improve cure rates without making graft-versus-host disease worse.”
The National Institutes of Health, the National Cancer Institute, an ASCO Young Investigator Award, the University Cancer Research Fund, and the Scott Neil Schwirck Fellowship funded the work.
Source: UNC-Chapel Hill


